Morphological plasticity of astroglia: Understanding synaptic microenvironment
- PMID: 25782611
- PMCID: PMC4737250
- DOI: 10.1002/glia.22821
Morphological plasticity of astroglia: Understanding synaptic microenvironment
Abstract
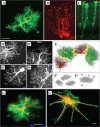
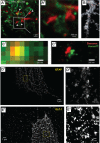
Memory formation in the brain is thought to rely on the remodeling of synaptic connections which eventually results in neural network rewiring. This remodeling is likely to involve ultrathin astroglial protrusions which often occur in the immediate vicinity of excitatory synapses. The phenomenology, cellular mechanisms, and causal relationships of such astroglial restructuring remain, however, poorly understood. This is in large part because monitoring and probing of the underpinning molecular machinery on the scale of nanoscopic astroglial compartments remains a challenge. Here we briefly summarize the current knowledge regarding the cellular organisation of astroglia in the synaptic microenvironment and discuss molecular mechanisms potentially involved in use-dependent astroglial morphogenesis. We also discuss recent observations concerning morphological astroglial plasticity, the respective monitoring methods, and some of the newly emerging techniques that might help with conceptual advances in the area.
Keywords: astrocyte plasticity; perisynaptic astrocytic processes; super-resolution microscopy.
© 2015 The Authors. Glia Published by Wiley Periodicals, Inc.
Figures



Similar articles
-
The role of astrocyte structural plasticity in regulating neural circuit function and behavior.Glia. 2022 Aug;70(8):1467-1483. doi: 10.1002/glia.24191. Epub 2022 May 10. Glia. 2022. PMID: 35535566 Free PMC article. Review.
-
Astroglial cradle in the life of the synapse.Philos Trans R Soc Lond B Biol Sci. 2014 Oct 19;369(1654):20130595. doi: 10.1098/rstb.2013.0595. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25225089 Free PMC article. Review.
-
Role of astroglia in estrogen regulation of synaptic plasticity and brain repair.J Neurobiol. 1999 Sep 15;40(4):574-84. J Neurobiol. 1999. PMID: 10453057 Review.
-
Perisynaptic astroglial processes: dynamic processors of neuronal information.Brain Struct Funct. 2016 Jun;221(5):2427-42. doi: 10.1007/s00429-015-1070-3. Epub 2015 May 31. Brain Struct Funct. 2016. PMID: 26026482 Review.
-
A Method to Visualize the Nanoscopic Morphology of Astrocytes In Vitro and In Situ.Methods Mol Biol. 2019;1938:69-84. doi: 10.1007/978-1-4939-9068-9_5. Methods Mol Biol. 2019. PMID: 30617973
Cited by
-
A Purinergic P2 Receptor Family-Mediated Increase in Thrombospondin-1 Bolsters Synaptic Density and Epileptic Seizure Activity in the Amygdala-Kindling Rat Model.Front Cell Neurosci. 2018 Oct 1;12:302. doi: 10.3389/fncel.2018.00302. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30386206 Free PMC article.
-
Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats.Anat Cell Biol. 2018 Mar;51(1):41-51. doi: 10.5115/acb.2018.51.1.41. Epub 2018 Mar 28. Anat Cell Biol. 2018. PMID: 29644109 Free PMC article.
-
Central Amygdala Astrocyte Plasticity Underlies GABAergic Dysregulation in Ethanol Dependence.bioRxiv [Preprint]. 2024 Jun 11:2024.06.11.598470. doi: 10.1101/2024.06.11.598470. bioRxiv. 2024. PMID: 38915577 Free PMC article. Preprint.
-
Control of Long-Term Plasticity by Glutamate Transporters.Front Synaptic Neurosci. 2019 Apr 9;11:10. doi: 10.3389/fnsyn.2019.00010. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31024287 Free PMC article. Review.
-
ECS Dynamism and Its Influence on Neuronal Excitability and Seizures.Neurochem Res. 2019 May;44(5):1020-1036. doi: 10.1007/s11064-019-02773-w. Epub 2019 Mar 16. Neurochem Res. 2019. PMID: 30879174 Review.
References
-
- Agulhon C, Fiacco TA, McCarthy KD. 2010. Hippocampal short‐ and long‐term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327:1250–1254. - PubMed
-
- Albrecht D, Lopez‐Murcia FJ, Perez‐Gonzalez AP, Lichtner G, Solsona C, Llobet A. 2012. SPARC prevents maturation of cholinergic presynaptic terminals. Mol Cell Neurosci 49:364–374. - PubMed
-
- Allen NJ. 2013. Role of glia in developmental synapse formation. Curr Opin Neurobiol 23:1027–1033. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

