Variable processing and cross-presentation of HIV by dendritic cells and macrophages shapes CTL immunodominance and immune escape
- PMID: 25781895
- PMCID: PMC4364612
- DOI: 10.1371/journal.ppat.1004725
Variable processing and cross-presentation of HIV by dendritic cells and macrophages shapes CTL immunodominance and immune escape
Abstract
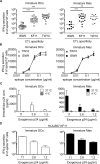
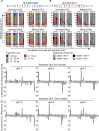
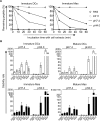
Dendritic cells (DCs) and macrophages (Møs) internalize and process exogenous HIV-derived antigens for cross-presentation by MHC-I to cytotoxic CD8⁺ T cells (CTL). However, how degradation patterns of HIV antigens in the cross-presentation pathways affect immunodominance and immune escape is poorly defined. Here, we studied the processing and cross-presentation of dominant and subdominant HIV-1 Gag-derived epitopes and HLA-restricted mutants by monocyte-derived DCs and Møs. The cross-presentation of HIV proteins by both DCs and Møs led to higher CTL responses specific for immunodominant epitopes. The low CTL responses to subdominant epitopes were increased by pretreatment of target cells with peptidase inhibitors, suggestive of higher intracellular degradation of the corresponding peptides. Using DC and Mø cell extracts as a source of cytosolic, endosomal or lysosomal proteases to degrade long HIV peptides, we identified by mass spectrometry cell-specific and compartment-specific degradation patterns, which favored the production of peptides containing immunodominant epitopes in all compartments. The intracellular stability of optimal HIV-1 epitopes prior to loading onto MHC was highly variable and sequence-dependent in all compartments, and followed CTL hierarchy with immunodominant epitopes presenting higher stability rates. Common HLA-associated mutations in a dominant epitope appearing during acute HIV infection modified the degradation patterns of long HIV peptides, reduced intracellular stability and epitope production in cross-presentation-competent cell compartments, showing that impaired epitope production in the cross-presentation pathway contributes to immune escape. These findings highlight the contribution of degradation patterns in the cross-presentation pathway to HIV immunodominance and provide the first demonstration of immune escape affecting epitope cross-presentation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Different antigen-processing activities in dendritic cells, macrophages, and monocytes lead to uneven production of HIV epitopes and affect CTL recognition.J Immunol. 2014 Nov 1;193(9):4322-4334. doi: 10.4049/jimmunol.1400491. Epub 2014 Sep 17. J Immunol. 2014. PMID: 25230751 Free PMC article.
-
Portable flanking sequences modulate CTL epitope processing.J Clin Invest. 2007 Nov;117(11):3563-75. doi: 10.1172/JCI32047. J Clin Invest. 2007. PMID: 17975674 Free PMC article.
-
Effects of Cross-Presentation, Antigen Processing, and Peptide Binding in HIV Evasion of T Cell Immunity.J Immunol. 2018 Mar 1;200(5):1853-1864. doi: 10.4049/jimmunol.1701523. Epub 2018 Jan 26. J Immunol. 2018. PMID: 29374075 Free PMC article.
-
Escape of human immunodeficiency virus from immune control.Annu Rev Immunol. 1997;15:271-96. doi: 10.1146/annurev.immunol.15.1.271. Annu Rev Immunol. 1997. PMID: 9143689 Review.
-
Antigen-presenting cells and the selection of immunodominant epitopes.Crit Rev Immunol. 1997;17(5-6):411-7. Crit Rev Immunol. 1997. PMID: 9419428 Review.
Cited by
-
Intratumoral delivery of TransCon™ TLR7/8 Agonist promotes sustained anti-tumor activity and local immune cell activation while minimizing systemic cytokine induction.Cancer Cell Int. 2022 Sep 19;22(1):286. doi: 10.1186/s12935-022-02708-6. Cancer Cell Int. 2022. PMID: 36123697 Free PMC article.
-
A natural polymorphism of Mycobacterium tuberculosis in the esxH gene disrupts immunodomination by the TB10.4-specific CD8 T cell response.PLoS Pathog. 2020 Oct 19;16(10):e1009000. doi: 10.1371/journal.ppat.1009000. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33075106 Free PMC article.
-
Antigen Cross-Presentation by Macrophages.Front Immunol. 2020 Jul 8;11:1276. doi: 10.3389/fimmu.2020.01276. eCollection 2020. Front Immunol. 2020. PMID: 32733446 Free PMC article. Review.
-
Pulmonary Dendritic Cell Subsets Shape the Respiratory Syncytial Virus-Specific CD8+ T Cell Immunodominance Hierarchy in Neonates.J Immunol. 2017 Jan 1;198(1):394-403. doi: 10.4049/jimmunol.1600486. Epub 2016 Nov 28. J Immunol. 2017. PMID: 27895172 Free PMC article.
-
Enhancement of Peptide Vaccine Immunogenicity by Increasing Lymphatic Drainage and Boosting Serum Stability.Cancer Immunol Res. 2018 Sep;6(9):1025-1038. doi: 10.1158/2326-6066.CIR-17-0607. Epub 2018 Jun 18. Cancer Immunol Res. 2018. PMID: 29915023 Free PMC article.
References
-
- Yewdell JW Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25: 533–543. - PubMed
-
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3: 205–211. - PubMed
-
- Allen TM, Altfeld M, Geer SC, Kalife ET, Moore C, O'Sullivan K M, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol. 2005;79: 13239–13249. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

