Mouse Ovarian Very Small Embryonic-Like Stem Cells Resist Chemotherapy and Retain Ability to Initiate Oocyte-Specific Differentiation
- PMID: 25779995
- PMCID: PMC4565482
- DOI: 10.1177/1933719115576727
Mouse Ovarian Very Small Embryonic-Like Stem Cells Resist Chemotherapy and Retain Ability to Initiate Oocyte-Specific Differentiation
Abstract
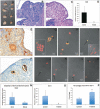
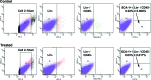
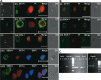
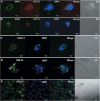
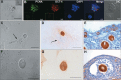
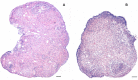
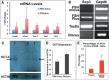
This study was undertaken to investigate stem cells in adult mouse ovary, the effect of chemotherapy on them and their potential to differentiate into germ cells. Very small embryonic-like stem cells (VSELs) that were SCA-1+/Lin-/CD45-, positive for nuclear octamer-binding transforming factor 4 (OCT-4), Nanog, and cell surface stage-specific embryonic antigen 1, were identified in adult mouse ovary. Chemotherapy resulted in complete loss of follicular reserve and cytoplasmic OCT-4 positive progenitors (ovarian germ stem cells) but VSELs survived. In ovarian surface epithelial (OSE) cell cultures from chemoablated ovary, proliferating germ cell clusters and mouse vasa homolog/growth differentiation factor 9-positive oocyte-like structure were observed by day 6, probably arising as a result of differentiation of the surviving VSELs. Follicle-stimulating hormone (FSH) exerted a direct stimulatory action on the OSE and induced stem cells proliferation and differentiation into premeiotic germ cell clusters during intact chemoablated ovaries culture. The FSH analog pregnant mare serum gonadotropin treatment to chemoablated mice increased the percentage of surviving VSELs in ovary. The results of this study provide evidence for the presence of potential VSELs in mouse ovaries and show that they survive chemotherapy, are modulated by FSH, and retain the ability to undergo oocyte-specific differentiation. These results show relevance to women who undergo premature ovarian failure because of oncotherapy.
Keywords: FSH; OCT-4; VSELs; ovary; stem cells.
© The Author(s) 2015.
Conflict of interest statement
Figures



Similar articles
-
Testicular Stem Cells Express Follicle-Stimulating Hormone Receptors and Are Directly Modulated by FSH.Reprod Sci. 2016 Nov;23(11):1493-1508. doi: 10.1177/1933719116643593. Epub 2016 May 6. Reprod Sci. 2016. PMID: 27189070
-
Delineating the effects of 5-fluorouracil and follicle-stimulating hormone on mouse bone marrow stem/progenitor cells.Stem Cell Res Ther. 2016 Apr 19;7(1):59. doi: 10.1186/s13287-016-0311-6. Stem Cell Res Ther. 2016. PMID: 27095238 Free PMC article.
-
Further characterization of adult sheep ovarian stem cells and their involvement in neo-oogenesis and follicle assembly.J Ovarian Res. 2018 Jan 5;11(1):3. doi: 10.1186/s13048-017-0377-5. J Ovarian Res. 2018. PMID: 29304868 Free PMC article.
-
Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead.Hum Reprod Update. 2016 Dec;23(1):41-76. doi: 10.1093/humupd/dmw030. Epub 2016 Sep 10. Hum Reprod Update. 2016. PMID: 27614362 Review.
-
Stem Cells in the Mammalian Gonads.Adv Exp Med Biol. 2019;1201:109-123. doi: 10.1007/978-3-030-31206-0_6. Adv Exp Med Biol. 2019. PMID: 31898784 Review.
Cited by
-
Used protocols for isolation and propagation of ovarian stem cells, different cells with different traits.J Ovarian Res. 2016 Oct 20;9(1):68. doi: 10.1186/s13048-016-0274-3. J Ovarian Res. 2016. PMID: 27765047 Free PMC article. Review.
-
Ovarian stem cells-resolving controversies.J Assist Reprod Genet. 2018 Mar;35(3):393-398. doi: 10.1007/s10815-017-1080-6. Epub 2017 Nov 11. J Assist Reprod Genet. 2018. PMID: 29128912 Free PMC article.
-
Novel Action of FSH on Stem Cells in Adult Mammalian Ovary Induces Postnatal Oogenesis and Primordial Follicle Assembly.Stem Cells Int. 2016;2016:5096596. doi: 10.1155/2016/5096596. Epub 2015 Nov 9. Stem Cells Int. 2016. PMID: 26635884 Free PMC article. Review.
-
Ovarian microenvironment: challenges and opportunities in protecting against chemotherapy-associated ovarian damage.Hum Reprod Update. 2024 Oct 1;30(5):614-647. doi: 10.1093/humupd/dmae020. Hum Reprod Update. 2024. PMID: 38942605 Free PMC article. Review.
-
Endogenous, tissue-resident stem/progenitor cells in gonads and bone marrow express FSHR and respond to FSH via FSHR-3.J Ovarian Res. 2021 Oct 30;14(1):145. doi: 10.1186/s13048-021-00883-0. J Ovarian Res. 2021. PMID: 34717703 Free PMC article. Review.
References
-
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428 (6979):145–150. - PubMed
-
- Pacchiarotti J, Maki C, Ramos T, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 2010;79 (3):159–170. - PubMed
-
- Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11 (5):631–636. - PubMed
-
- Zhang D, Fouad H, Zoma WD, Salama SA, Wentz MJ, Al-Hendy A. Expression of stem and germ cell markers within nonfollicle structures in adult mouse ovary. Reprod Sci. 2008;15 (2):139–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

