Metabolic pathways promoting cancer cell survival and growth
- PMID: 25774832
- PMCID: PMC4939711
- DOI: 10.1038/ncb3124
Metabolic pathways promoting cancer cell survival and growth
Abstract
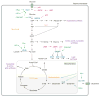
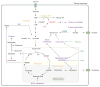
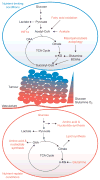
Activation of oncogenes and loss of tumour suppressors promote metabolic reprogramming in cancer, resulting in enhanced nutrient uptake to supply energetic and biosynthetic pathways. However, nutrient limitations within solid tumours may require that malignant cells exhibit metabolic flexibility to sustain growth and survival. Here, we highlight these adaptive mechanisms and also discuss emerging approaches to probe tumour metabolism in vivo and their potential to expand the metabolic repertoire of malignant cells even further.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells.Int J Biochem Cell Biol. 2011 Jul;43(7):950-68. doi: 10.1016/j.biocel.2010.05.003. Epub 2010 May 10. Int J Biochem Cell Biol. 2011. PMID: 20460169 Review.
-
The Role of Glucose and Lipid Metabolism in Growth and Survival of Cancer Cells.Recent Results Cancer Res. 2016;207:1-22. doi: 10.1007/978-3-319-42118-6_1. Recent Results Cancer Res. 2016. PMID: 27557532 Review.
-
Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis.Semin Cell Dev Biol. 2012 Jun;23(4):362-9. doi: 10.1016/j.semcdb.2012.02.002. Epub 2012 Feb 11. Semin Cell Dev Biol. 2012. PMID: 22349059 Review.
-
Metabolic transformation in cancer.Carcinogenesis. 2009 Aug;30(8):1269-80. doi: 10.1093/carcin/bgp070. Epub 2009 Mar 25. Carcinogenesis. 2009. PMID: 19321800 Review.
-
Glucose avidity of carcinomas.Cancer Lett. 2009 Apr 18;276(2):125-35. doi: 10.1016/j.canlet.2008.08.007. Epub 2008 Sep 14. Cancer Lett. 2009. PMID: 18790562 Review.
Cited by
-
Resensitizing Paclitaxel-Resistant Ovarian Cancer via Targeting Lipid Metabolism Key Enzymes CPT1A, SCD and FASN.Int J Mol Sci. 2023 Nov 19;24(22):16503. doi: 10.3390/ijms242216503. Int J Mol Sci. 2023. PMID: 38003694 Free PMC article.
-
How DNA methylation affects the Warburg effect.Int J Biol Sci. 2020 Apr 27;16(12):2029-2041. doi: 10.7150/ijbs.45420. eCollection 2020. Int J Biol Sci. 2020. PMID: 32549751 Free PMC article. Review.
-
Enzymes for N-Glycan Branching and Their Genetic and Nongenetic Regulation in Cancer.Biomolecules. 2016 Apr 28;6(2):25. doi: 10.3390/biom6020025. Biomolecules. 2016. PMID: 27136596 Free PMC article. Review.
-
PML at Mitochondria-Associated Membranes Is Critical for the Repression of Autophagy and Cancer Development.Cell Rep. 2016 Aug 30;16(9):2415-27. doi: 10.1016/j.celrep.2016.07.082. Epub 2016 Aug 18. Cell Rep. 2016. PMID: 27545895 Free PMC article.
-
The Role of Tumor Metabolic Reprogramming in Tumor Immunity.Int J Mol Sci. 2023 Dec 13;24(24):17422. doi: 10.3390/ijms242417422. Int J Mol Sci. 2023. PMID: 38139250 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

