Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins
- PMID: 25774711
- PMCID: PMC4382692
- DOI: 10.1038/ncomms7490
Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins
Abstract
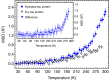
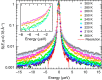
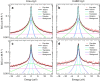
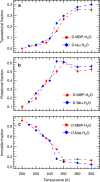
Hydration water is the natural matrix of biological macromolecules and is essential for their activity in cells. The coupling between water and protein dynamics has been intensively studied, yet it remains controversial. Here we combine protein perdeuteration, neutron scattering and molecular dynamics simulations to explore the nature of hydration water motions at temperatures between 200 and 300 K, across the so-called protein dynamical transition, in the intrinsically disordered human protein tau and the globular maltose binding protein. Quasi-elastic broadening is fitted with a model of translating, rotating and immobile water molecules. In both experiment and simulation, the translational component markedly increases at the protein dynamical transition (around 240 K), regardless of whether the protein is intrinsically disordered or folded. Thus, we generalize the notion that the translational diffusion of water molecules on a protein surface promotes the large-amplitude motions of proteins that are required for their biological activity.
Figures
Similar articles
-
Dynamical coupling of intrinsically disordered proteins and their hydration water: comparison with folded soluble and membrane proteins.Biophys J. 2012 Jul 3;103(1):129-36. doi: 10.1016/j.bpj.2012.05.027. Biophys J. 2012. PMID: 22828339 Free PMC article.
-
Diffusion of Hydration Water around Intrinsically Disordered Proteins.J Phys Chem B. 2015 Oct 22;119(42):13262-70. doi: 10.1021/acs.jpcb.5b07248. Epub 2015 Oct 8. J Phys Chem B. 2015. PMID: 26418258
-
Local Structure and Dynamics of Hydration Water in Intrinsically Disordered Proteins.J Phys Chem B. 2015 Aug 27;119(34):10858-67. doi: 10.1021/jp511961c. Epub 2015 Apr 22. J Phys Chem B. 2015. PMID: 25871264
-
Dynamics of hydration water in proteins.Gen Physiol Biophys. 2009 Jun;28(2):168-73. doi: 10.4149/gpb_2009_02_168. Gen Physiol Biophys. 2009. PMID: 19592713 Review.
-
Force field development and simulations of intrinsically disordered proteins.Curr Opin Struct Biol. 2018 Feb;48:40-48. doi: 10.1016/j.sbi.2017.10.008. Epub 2017 Nov 5. Curr Opin Struct Biol. 2018. PMID: 29080468 Free PMC article. Review.
Cited by
-
Competing coexisting phases in 2D water.Sci Rep. 2016 May 17;6:25938. doi: 10.1038/srep25938. Sci Rep. 2016. PMID: 27185018 Free PMC article.
-
Decoupling of the onset of anharmonicity between a protein and its surface water around 200 K.Elife. 2024 Aug 19;13:RP95665. doi: 10.7554/eLife.95665. Elife. 2024. PMID: 39158544 Free PMC article.
-
Investigation of the Dynamic Behaviour of H2 and D2 in a Kinetic Quantum Sieving System.ACS Appl Mater Interfaces. 2024 Mar 13;16(10):12467-12478. doi: 10.1021/acsami.3c17965. Epub 2024 Feb 29. ACS Appl Mater Interfaces. 2024. PMID: 38423989 Free PMC article.
-
Ion influence on surface water dynamics and proton exchange at protein surfaces - A unified model for transverse and longitudinal NMR relaxation dispersion.J Mol Liq. 2022 Dec 1;367(Pt A):120451. doi: 10.1016/j.molliq.2022.120451. Epub 2022 Sep 24. J Mol Liq. 2022. PMID: 37790165 Free PMC article.
-
A Quantitative Comparison of the Counting Significance of van Hove Integral Spectroscopy and Quasielastic Neutron Scattering.Sci Rep. 2020 Apr 14;10(1):6350. doi: 10.1038/s41598-020-63193-3. Sci Rep. 2020. PMID: 32286403 Free PMC article.
References
-
- Ball P. Water as an active constituent in cell biology. Chem. Rev. 108, 74–108 (2008) . - PubMed
-
- Rupley J. A. & Careri G. in Advances in Protein Chemistry Vol. 41, eds Anfinsen C. B., Edsall J. T., Richards F. M., Eisenberg D. S. 37–172Academic Press (1991) . - PubMed
-
- Doster W. & Settles M. Protein-water displacement distributions. Biochim. Biophys. Acta 1749, 173–186 (2005) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

