Mycophenolate mofetil therapy for steroid-resistant IgA nephropathy with the nephrotic syndrome in children
- PMID: 25773534
- PMCID: PMC4446504
- DOI: 10.1007/s00467-014-3041-y
Mycophenolate mofetil therapy for steroid-resistant IgA nephropathy with the nephrotic syndrome in children
Abstract
Background: Immunoglobulin A nephropathy (IgAN) presents as nephrotic syndrome (NS) relatively rarely, and the current treatment experience of IgAN patients with NS is mostly with adults. The objective of our study was to investigate the efficacy of corticosteroids and mycophenolate mofetil (MMF) in treating childhood immunoglobulin A nephropathy (IgAN) with nephrotic syndrome.
Methods: A total of 58 children (39 boys and 19 girls) diagnosed with nephrotic syndrome and primary IgAN were enrolled in the study. All the patients were administered prednisone 2 mg/kg per day for 8 weeks. Steroid-resistant patients were treated with the combined use of MMF (dose of 20 ~ 30 mg/kg per day) and prednisone for 6-12 months. The prednisone dose was reduced stepwise during the combined treatment.
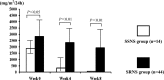
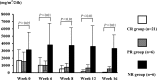
Results: Of the 58 children, 14 were steroid-sensitive (M, S, and T variants of the Oxford classification were 0 in most children), and 44 cases who presented serious pathological damage to the kidney were steroid-resistant. The estimated glomerular filtration rate (eGFR) of the steroid-resistant children (86.69 ± 26.85 ml/min/1.73 m(2)) was significantly lower (P < 0.05) than that of the steroid-sensitive children (106.89 ± 26.94 ml/min/1.73 m(2)). After 4 months of combined MMF treatment in 33 steroid-resistant children, complete remission of proteinuria was found in 21 cases, partial remission of proteinuria in 6 cases, and no response was found in 6 cases. Except for the T variant, other variants of the Oxford classification, including M, E, and S morphological variables, was not significantly different among patients complete remission, those with partial remission, and those with no response. The eGFR of children with complete remission of proteinuria (100.04 ± 18.47 ml/min/1.73 m(2)), that of those with partial remission (92.24 ± 27.63 ml/min/1.73 m(2)), and that of those with no response (72.17 ± 27.55 ml/min/1.73 m(2)) were significantly different (P < 0.05).
Conclusion: Corticosteroid therapy showed satisfactory efficacy in IgAN children with nephrotic syndrome and slight pathological damage. The effect of MMF was good for steroid-resistant IgAN children, but poor for those with tubular atrophy/interstitial fibrosis and renal function impairment.
Figures
Similar articles
-
Mycophenolate Mofetil Combined With Prednisone Versus Full-Dose Prednisone in IgA Nephropathy With Active Proliferative Lesions: A Randomized Controlled Trial.Am J Kidney Dis. 2017 Jun;69(6):788-795. doi: 10.1053/j.ajkd.2016.11.027. Epub 2017 Feb 16. Am J Kidney Dis. 2017. PMID: 28215945 Clinical Trial.
-
[Mycophenolate mofetil in treatment of childhood nephrotic syndrome--preliminary report].Przegl Lek. 2006;63 Suppl 3:44-8. Przegl Lek. 2006. PMID: 16898486 Clinical Trial. Polish.
-
A comparison of a standard-dose prednisone regimen and mycophenolate mofetil combined with a lower prednisone dose in Chinese adults with idiopathic nephrotic syndrome who were carriers of hepatitis B surface antigen: a prospective cohort study.Clin Ther. 2009 Apr;31(4):741-50. doi: 10.1016/j.clinthera.2009.04.011. Clin Ther. 2009. PMID: 19446147 Clinical Trial.
-
Treatment of IgA nephropathy: corticosteroids, tonsillectomy, and mycophenolate mofetil.Contrib Nephrol. 2007;157:37-43. doi: 10.1159/000102286. Contrib Nephrol. 2007. PMID: 17495435 Review.
-
New treatment strategies in idiopathic nephrotic syndrome.Minerva Pediatr. 2012 Apr;64(2):135-43. Minerva Pediatr. 2012. PMID: 22495188 Review.
Cited by
-
The Emerging Role of Pathogenesis of IgA Nephropathy.J Clin Med. 2018 Aug 20;7(8):225. doi: 10.3390/jcm7080225. J Clin Med. 2018. PMID: 30127305 Free PMC article. Review.
-
Nephropathy in a Child with Severe Recessive Dystrophic Epidermolysis Bullosa Treated with Cyclophosphamide: A Case Report.Case Rep Nephrol Dial. 2023 Jul 14;13(1):75-83. doi: 10.1159/000530875. eCollection 2023 Jan-Dec. Case Rep Nephrol Dial. 2023. PMID: 37484797 Free PMC article.
-
IPNA clinical practice recommendations for the diagnosis and management of children with IgA nephropathy and IgA vasculitis nephritis.Pediatr Nephrol. 2025 Feb;40(2):533-569. doi: 10.1007/s00467-024-06502-6. Epub 2024 Sep 27. Pediatr Nephrol. 2025. PMID: 39331079 Free PMC article.
-
Why, when and how should immunosuppressive therapy considered in patients with immunoglobulin A nephropathy?Clin Exp Immunol. 2016 Nov;186(2):115-133. doi: 10.1111/cei.12823. Epub 2016 Sep 8. Clin Exp Immunol. 2016. PMID: 27283488 Free PMC article. Review.
-
Nephrotic syndrome is a rare manifestation of IGA nephropathy.Int J Health Sci (Qassim). 2016 Jul;10(3):455-8. Int J Health Sci (Qassim). 2016. PMID: 27610069 Free PMC article.
References
-
- Radford MG, Jr, Donadio JV, Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

