Rat Cytomegalovirus Vaccine Prevents Accelerated Chronic Rejection in CMV-Naïve Recipients of Infected Donor Allograft Hearts
- PMID: 25766876
- PMCID: PMC5006870
- DOI: 10.1111/ajt.13188
Rat Cytomegalovirus Vaccine Prevents Accelerated Chronic Rejection in CMV-Naïve Recipients of Infected Donor Allograft Hearts
Abstract
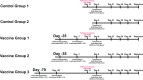
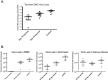
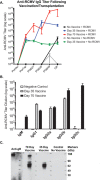
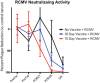
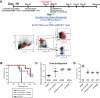
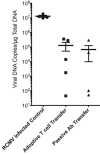
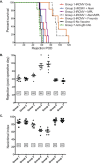
Cytomegalovirus accelerates transplant vascular sclerosis (TVS) and chronic rejection (CR) in solid organ transplants; however, the mechanisms involved are unclear. We determined the efficacy of a CMV vaccine in preventing CMV-accelerated rat cardiac allograft rejection in naïve recipients of CMV+ donor hearts. F344 donor rats were infected with RCMV 5 days prior to heterotopic cardiac transplantation into CMV-naïve or H2 O2 -inactivated RCMV-vaccinated Lewis recipients. Recipients of RCMV-infected donor hearts rejected at POD59, whereas vaccinated recipients exhibited a significantly prolonged time to rejection-POD97, similar to recipients of uninfected donor hearts (POD108). Although all of the donor hearts were preinfected, the vaccinated recipients had lower graft and PBMC viral loads at POD 7 compared to unvaccinated controls. Adoptive T cell and passive antibody transfers from vaccinated Lewis rats into naïve recipients demonstrate that both T-cell and B-cell arms of the adaptive immune response provide protection against CMV-accelerated rejection. Similar findings were obtained when testing three different adjuvants in passive transfer experiments. We have determined that the timing of the vaccine prior to transplantation and the specific adjuvant play critical roles in mediating anti-viral responses and promoting graft survival. CMV vaccination prior to transplantation may effectively increase graft survival.
Keywords: animal models; basic (laboratory) research / science; graft survival; heart transplantation / cardiology; infection and infectious agents; infectious disease; vaccine; viral: Cytomegalovirus (CMV).
© Copyright 2015 The Authors. American Journal of Transplantation published by Wiley Periodicals Inc.
Figures

Similar articles
-
Macrophage depletion of CMV latently infected donor hearts ameliorates recipient accelerated chronic rejection.Transpl Infect Dis. 2021 Apr;23(2):e13514. doi: 10.1111/tid.13514. Epub 2020 Dec 7. Transpl Infect Dis. 2021. PMID: 33205500 Free PMC article.
-
Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants.Transplantation. 2002 Mar 15;73(5):679-88. doi: 10.1097/00007890-200203150-00005. Transplantation. 2002. PMID: 11907411
-
Cytomegalovirus latency promotes cardiac lymphoid neogenesis and accelerated allograft rejection in CMV naïve recipients.Am J Transplant. 2011 Jan;11(1):45-55. doi: 10.1111/j.1600-6143.2010.03365.x. Am J Transplant. 2011. PMID: 21199347 Free PMC article.
-
Cytomegalovirus infection and cardiac allograft vasculopathy.Transpl Infect Dis. 1999 Jun;1(2):115-26. doi: 10.1034/j.1399-3062.1999.010205.x. Transpl Infect Dis. 1999. PMID: 11428979 Review.
-
Revisiting the effects of CMV on long-term transplant outcome.Curr Opin Organ Transplant. 2010 Aug;15(4):492-8. doi: 10.1097/MOT.0b013e32833bd3b5. Curr Opin Organ Transplant. 2010. PMID: 20631617 Review.
Cited by
-
Disruption of Transplant Tolerance by an "Incognito" Form of CD8 T Cell-Dependent Memory.Am J Transplant. 2017 Jul;17(7):1742-1753. doi: 10.1111/ajt.14194. Epub 2017 Feb 21. Am J Transplant. 2017. PMID: 28066981 Free PMC article.
-
Macrophage depletion of CMV latently infected donor hearts ameliorates recipient accelerated chronic rejection.Transpl Infect Dis. 2021 Apr;23(2):e13514. doi: 10.1111/tid.13514. Epub 2020 Dec 7. Transpl Infect Dis. 2021. PMID: 33205500 Free PMC article.
-
Maternal Immunity and the Natural History of Congenital Human Cytomegalovirus Infection.Viruses. 2018 Aug 3;10(8):405. doi: 10.3390/v10080405. Viruses. 2018. PMID: 30081449 Free PMC article. Review.
-
Development of a next-generation chikungunya virus vaccine based on the HydroVax platform.PLoS Pathog. 2022 Jul 5;18(7):e1010695. doi: 10.1371/journal.ppat.1010695. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35788221 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
References
-
- Bruning JH, Persoons MCJ, Lemstrom KB, Stals FS, De Clereq E, Bruggeman CA. Enhancement of transplantation associated atherosclerosis by CMV, which can be prevented by antiviral therapy in the form of HPMPC. Transplant Int 1994; 7: 365–370. - PubMed
-
- Lemstrom K, Koskinen P, Krogerus L, Daemen M, Bruggeman CA, Hayry PJ. Cytomegalovirus antigen expression, endothelial cell proliferation, and intimal thickening in rat cardiac allografts after cytomegalovirus infection. Circ 1995; 92: 2594–2604. - PubMed
-
- Orloff SL. Elimination of donor‐specific alloreactivity by bone marrow chimerism prevents cytomegalovirus accelerated transplant vascular sclerosis in rat small bowel transplants. J Clin Virol 1999; 12: 142 (G3–25).
-
- Orloff SL, Streblow DN, Soderberg‐Naucler C, et al. Elimination of donor‐specific alloreactivity prevents cytomegalovirus‐accelerated chronic rejection in rat small bowel and heart transplants. Transplantation 2002; 73: 679–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

