Membrane-initiated non-genomic signaling by estrogens in the hypothalamus: cross-talk with glucocorticoids with implications for behavior
- PMID: 25762980
- PMCID: PMC4329805
- DOI: 10.3389/fendo.2015.00018
Membrane-initiated non-genomic signaling by estrogens in the hypothalamus: cross-talk with glucocorticoids with implications for behavior
Abstract
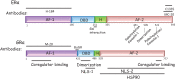
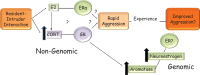
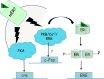
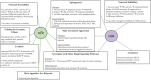
The estrogen receptor and glucocorticoid receptor are members of the nuclear receptor superfamily that can signal using both non-genomic and genomic transcriptional modes. Though genomic modes of signaling have been well characterized and several behaviors attributed to this signaling mechanism, the physiological significance of non-genomic modes of signaling has not been well understood. This has partly been due to the controversy regarding the identity of the membrane ER (mER) or membrane GR (mGR) that may mediate rapid, non-genomic signaling and the downstream signaling cascades that may result as a consequence of steroid ligands binding the mER or the mGR. Both estrogens and glucocorticoids exert a number of actions on the hypothalamus, including feedback. This review focuses on the various candidates for the mER or mGR in the hypothalamus and the contribution of non-genomic signaling to classical hypothalamically driven behaviors and changes in neuronal morphology. It also attempts to categorize some of the possible functions of non-genomic signaling at both the cellular level and at the organismal level that are relevant for behavior, including some behaviors that are regulated by both estrogens and glucocorticoids in a potentially synergistic manner. Lastly, it attempts to show that steroid signaling via non-genomic modes may provide the organism with rapid behavioral responses to stimuli.
Keywords: GPCR; aggression; estrogen receptor variants; glucocorticoid receptor; hypothalamus; lordosis; membrane-initiated signaling; spine density.
Figures
Similar articles
-
Estrogen signaling in the hypothalamus.Vitam Horm. 2005;71:123-45. doi: 10.1016/S0083-6729(05)71005-0. Vitam Horm. 2005. PMID: 16112267 Review.
-
Non-genomic actions of estrogens and their interaction with genomic actions in the brain.Front Neuroendocrinol. 2008 May;29(2):238-57. doi: 10.1016/j.yfrne.2007.08.003. Epub 2007 Oct 1. Front Neuroendocrinol. 2008. PMID: 18083219 Review.
-
Integration of steroid hormone initiated membrane action to genomic function in the brain.Steroids. 2005 May-Jun;70(5-7):388-96. doi: 10.1016/j.steroids.2005.02.007. Steroids. 2005. PMID: 15862822 Review.
-
Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles.Endocr Rev. 2007 Feb;28(1):1-19. doi: 10.1210/er.2005-0021. Epub 2006 Oct 3. Endocr Rev. 2007. PMID: 17018839 Review.
-
Minireview: estrogen receptor-mediated rapid signaling.Endocrinology. 2006 Dec;147(12):5557-63. doi: 10.1210/en.2006-0729. Epub 2006 Aug 31. Endocrinology. 2006. PMID: 16946015 Review.
Cited by
-
Activation of the G Protein-Coupled Estrogen Receptor Elicits Store Calcium Release and Phosphorylation of the Mu-Opioid Receptors in the Human Neuroblastoma SH-SY5Y Cells.Front Neurosci. 2019 Dec 17;13:1351. doi: 10.3389/fnins.2019.01351. eCollection 2019. Front Neurosci. 2019. PMID: 31920512 Free PMC article.
-
Estrogen Receptor Function: Impact on the Human Endometrium.Front Endocrinol (Lausanne). 2022 Feb 28;13:827724. doi: 10.3389/fendo.2022.827724. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35295981 Free PMC article. Review.
-
Membrane-Associated Effects of Glucocorticoid on BACE1 Upregulation and Aβ Generation: Involvement of Lipid Raft-Mediated CREB Activation.J Neurosci. 2017 Aug 30;37(35):8459-8476. doi: 10.1523/JNEUROSCI.0074-17.2017. Epub 2017 Aug 3. J Neurosci. 2017. PMID: 28855330 Free PMC article.
-
Temporal and bidirectional influences of estradiol on voluntary wheel running in adult female and male rats.Horm Behav. 2020 Apr;120:104694. doi: 10.1016/j.yhbeh.2020.104694. Epub 2020 Jan 27. Horm Behav. 2020. PMID: 31978389 Free PMC article.
-
Single-cell immunoblotting resolves estrogen receptor-α isoforms in breast cancer.PLoS One. 2021 Jul 27;16(7):e0254783. doi: 10.1371/journal.pone.0254783. eCollection 2021. PLoS One. 2021. PMID: 34314438 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

