Group VIA Phospholipase A2 (iPLA2β) Modulates Bcl-x 5'-Splice Site Selection and Suppresses Anti-apoptotic Bcl-x(L) in β-Cells
- PMID: 25762722
- PMCID: PMC4409262
- DOI: 10.1074/jbc.M115.648956
Group VIA Phospholipase A2 (iPLA2β) Modulates Bcl-x 5'-Splice Site Selection and Suppresses Anti-apoptotic Bcl-x(L) in β-Cells
Abstract
Diabetes is a consequence of reduced β-cell function and mass, due to β-cell apoptosis. Endoplasmic reticulum (ER) stress is induced during β-cell apoptosis due to various stimuli, and our work indicates that group VIA phospholipase A2β (iPLA2β) participates in this process. Delineation of underlying mechanism(s) reveals that ER stress reduces the anti-apoptotic Bcl-x(L) protein in INS-1 cells. The Bcl-x pre-mRNA undergoes alternative pre-mRNA splicing to generate Bcl-x(L) or Bcl-x(S) mature mRNA. We show that both thapsigargin-induced and spontaneous ER stress are associated with reductions in the ratio of Bcl-x(L)/Bcl-x(S) mRNA in INS-1 and islet β-cells. However, chemical inactivation or knockdown of iPLA2β augments the Bcl-x(L)/Bcl-x(S) ratio. Furthermore, the ratio is lower in islets from islet-specific RIP-iPLA2β transgenic mice, whereas islets from global iPLA2β(-/-) mice exhibit the opposite phenotype. In view of our earlier reports that iPLA2β induces ceramide accumulation through neutral sphingomyelinase 2 and that ceramides shift the Bcl-x 5'-splice site (5'SS) selection in favor of Bcl-x(S), we investigated the potential link between Bcl-x splicing and the iPLA2β/ceramide axis. Exogenous C6-ceramide did not alter Bcl-x 5'SS selection in INS-1 cells, and neutral sphingomyelinase 2 inactivation only partially prevented the ER stress-induced shift in Bcl-x splicing. In contrast, 5(S)-hydroxytetraenoic acid augmented the ratio of Bcl-x(L)/Bcl-x(S) by 15.5-fold. Taken together, these data indicate that β-cell apoptosis is, in part, attributable to the modulation of 5'SS selection in the Bcl-x pre-mRNA by bioactive lipids modulated by iPLA2β.
Keywords: Alternative Splicing; Apoptosis; Bcl-x; Diabetes; Phospholipase A; Signal Transduction; β-Cell.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



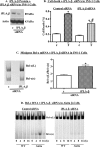
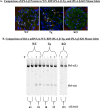

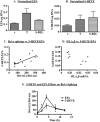
Similar articles
-
The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase.Biochemistry. 2007 Sep 4;46(35):10170-85. doi: 10.1021/bi700017z. Epub 2007 Aug 9. Biochemistry. 2007. PMID: 17685585 Free PMC article.
-
Genetic modulation of islet β-cell iPLA₂β expression provides evidence for its impact on β-cell apoptosis and autophagy.Islets. 2013 Jan-Feb;5(1):29-44. doi: 10.4161/isl.23758. Epub 2013 Jan 1. Islets. 2013. PMID: 23411472 Free PMC article.
-
Evidence of contribution of iPLA2β-mediated events during islet β-cell apoptosis due to proinflammatory cytokines suggests a role for iPLA2β in T1D development.Endocrinology. 2014 Sep;155(9):3352-64. doi: 10.1210/en.2013-2134. Epub 2014 Jul 8. Endocrinology. 2014. PMID: 25004092 Free PMC article.
-
A link between endoplasmic reticulum stress-induced β-cell apoptosis and the group VIA Ca2+-independent phospholipase A2 (iPLA2β).Diabetes Obes Metab. 2010 Oct;12 Suppl 2(0 2):93-8. doi: 10.1111/j.1463-1326.2010.01270.x. Diabetes Obes Metab. 2010. PMID: 21029305 Free PMC article. Review.
-
Group VIA Ca2+-independent phospholipase A2 (iPLA2beta) and its role in beta-cell programmed cell death.Biochimie. 2010 Jun;92(6):627-37. doi: 10.1016/j.biochi.2010.01.005. Epub 2010 Jan 18. Biochimie. 2010. PMID: 20083151 Free PMC article. Review.
Cited by
-
iPLA2β and its role in male fertility, neurological disorders, metabolic disorders, and inflammation.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):846-860. doi: 10.1016/j.bbalip.2018.10.010. Epub 2018 Nov 5. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30408523 Free PMC article. Review.
-
Regulation of β-cell death by ADP-ribosylhydrolase ARH3 via lipid signaling in insulitis.Cell Commun Signal. 2024 Feb 21;22(1):141. doi: 10.1186/s12964-023-01437-1. Cell Commun Signal. 2024. PMID: 38383396 Free PMC article.
-
Lysophosphatidylserine induces necrosis in pressure overloaded male mouse hearts via G protein coupled receptor 34.Nat Commun. 2023 Jul 31;14(1):4494. doi: 10.1038/s41467-023-40201-4. Nat Commun. 2023. PMID: 37524709 Free PMC article.
-
Calcium-independent phospholipases A2 and their roles in biological processes and diseases.J Lipid Res. 2015 Sep;56(9):1643-68. doi: 10.1194/jlr.R058701. Epub 2015 May 28. J Lipid Res. 2015. PMID: 26023050 Free PMC article. Review.
-
Serine/Arginine-Rich Splicing Factor 3 Modulates the Alternative Splicing of Cytoplasmic Polyadenylation Element Binding Protein 2.Mol Cancer Res. 2019 Sep;17(9):1920-1930. doi: 10.1158/1541-7786.MCR-18-1291. Epub 2019 May 28. Mol Cancer Res. 2019. PMID: 31138601 Free PMC article.
References
-
- Socha L., Silva D., Lesage S., Goodnow C., Petrovsky N. (2003) The role of endoplasmic reticulum stress in nonimmune diabetes: NOD.k iHEL, a novel model of β-cell death. Ann. N.Y. Acad. Sci. 1005, 178–183 - PubMed
-
- Delépine M., Nicolino M., Barrett T., Golamaully M., Lathrop G. M., Julier C. (2000) EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet. 25, 406–409 - PubMed
-
- Yamada T., Ishihara H., Tamura A., Takahashi R., Yamaguchi S., Takei D., Tokita A., Satake C., Tashiro F., Katagiri H., Aburatani H., Miyazaki J., Oka Y. (2006) WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum. Mol. Genet. 15, 1600–1609 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- KL2 TR000057/TR/NCATS NIH HHS/United States
- R01 DK069455/DK/NIDDK NIH HHS/United States
- NH1C06-RR17393/NH/NIH HHS/United States
- R01 HL125353/HL/NHLBI NIH HHS/United States
- I01 BX001792/BX/BLRD VA/United States
- HL072925/HL/NHLBI NIH HHS/United States
- R01 CA154314/CA/NCI NIH HHS/United States
- HL91388/HL/NHLBI NIH HHS/United States
- UL1 TR000058/TR/NCATS NIH HHS/United States
- R21 HL091388/HL/NHLBI NIH HHS/United States
- CA154314/CA/NCI NIH HHS/United States
- DK-69455/DK/NIDDK NIH HHS/United States
- R01 HL072925/HL/NHLBI NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- S10 OD010641/OD/NIH HHS/United States
- C06 RR017393/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

