New insights into the use of currently available non-steroidal anti-inflammatory drugs
- PMID: 25759598
- PMCID: PMC4346004
- DOI: 10.2147/JPR.S75160
New insights into the use of currently available non-steroidal anti-inflammatory drugs
Abstract
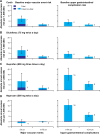
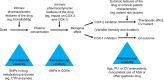
Non-steroidal anti-inflammatory drugs (NSAIDs), which act via inhibition of the cyclooxygenase (COX) isozymes, were discovered more than 100 years ago. They remain a key component of the pharmacological management of acute and chronic pain. The COX-1 and COX-2 isozymes have different biological functions; analgesic activity is primarily (although not exclusively) associated with inhibition of COX-2, while different side effects result from the inhibition of COX-1 and COX-2. All available NSAIDs, including acetaminophen and aspirin, are associated with potential side effects, particularly gastrointestinal and cardiovascular effects, related to their relative selectivity for COX-1 and COX-2. Since all NSAIDs exert their therapeutic activity through inhibition of the COX isozymes, strategies are needed to reduce the risks associated with NSAIDs while achieving sufficient pain relief. A better understanding of the inhibitory activity and COX-1/COX-2 selectivity of an NSAID at therapeutic doses, based on pharmacokinetic and pharmacodynamic properties (eg, inhibitory dose, absorption, plasma versus tissue distribution, and elimination), and the impact on drug tolerability and safety can guide the selection of appropriate NSAIDs for pain management. For example, many NSAIDs with moderate to high selectivity for COX-2 versus COX-1 can be administered at doses that maximize efficacy (~80% inhibition of COX-2) while minimizing COX-1 inhibition and associated side effects, such as gastrointestinal toxicity. Acidic NSAIDs with favorable tissue distribution and short plasma half-lives can additionally be dosed to provide near-constant analgesia while minimizing plasma concentrations to permit recovery of COX-mediated prostaglandin production in the vascular wall and other organs. Each patient's clinical background, including gastrointestinal and cardiovascular risk factors, should be taken into account when selecting appropriate NSAIDs. New methods are emerging to assist clinicians in the selection of appropriate NSAIDs and their doses/schedules, such as biomarkers that may predict the response to NSAID treatment in individual patients.
Keywords: cyclooxygenase inhibitors; cyclooxygenase selectivity; diclofenac; pain therapy; pharmacodynamics; pharmacokinetics.
Figures
Similar articles
-
Emerging evidence in NSAID pharmacology: important considerations for product selection.Am J Manag Care. 2015 Apr;21(7 Suppl):S139-47. Am J Manag Care. 2015. PMID: 26168321
-
Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest.J Vet Pharmacol Ther. 2004 Dec;27(6):479-90. doi: 10.1111/j.1365-2885.2004.00617.x. J Vet Pharmacol Ther. 2004. PMID: 15601442 Review.
-
[Preferential COX-2 inhibition: its clinical relevance for gastrointestinal non-steroidal anti-inflammatory rheumatic drug toxicity].Z Gastroenterol. 1999 Jan;37(1):45-58. Z Gastroenterol. 1999. PMID: 10091284 Review. German.
-
Anti-inflammatory drugs in the 21st century.Subcell Biochem. 2007;42:3-27. doi: 10.1007/1-4020-5688-5_1. Subcell Biochem. 2007. PMID: 17612044 Review.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.Health Technol Assess. 2008 Apr;12(11):1-278, iii. doi: 10.3310/hta12110. Health Technol Assess. 2008. PMID: 18405470 Review.
Cited by
-
National drug utilization trend of analgesics in China: an analysis of procurement data at 793 public hospitals from 2013 to 2018.J Pharm Policy Pract. 2021 May 25;14(1):45. doi: 10.1186/s40545-021-00325-8. J Pharm Policy Pract. 2021. PMID: 34034830 Free PMC article.
-
The Long-Term Safety of S-Flurbiprofen Plaster for Osteoarthritis Patients: An Open-Label, 52-Week Study.Clin Drug Investig. 2016 Aug;36(8):673-82. doi: 10.1007/s40261-016-0412-0. Clin Drug Investig. 2016. PMID: 27229525 Free PMC article. Clinical Trial.
-
Cys-loop receptors on cannabinoids: All high?Front Physiol. 2022 Nov 9;13:1044575. doi: 10.3389/fphys.2022.1044575. eCollection 2022. Front Physiol. 2022. PMID: 36439263 Free PMC article.
-
The effective interplay of (non-) selective NSAIDs with neostigmine in animal models of analgesia and inflammation.BMC Pharmacol Toxicol. 2021 May 1;22(1):24. doi: 10.1186/s40360-021-00488-9. BMC Pharmacol Toxicol. 2021. PMID: 33933169 Free PMC article.
-
Self-medication, self-assessment and knowledge of dental medicine students about analgesics.J Clin Exp Dent. 2024 Aug 1;16(8):e967-e974. doi: 10.4317/jced.61839. eCollection 2024 Aug. J Clin Exp Dent. 2024. PMID: 39281787 Free PMC article.
References
-
- Committee on Advancing Pain Research, Care, and Education; Institute of Medicine . Relieving Pain in America. Washington, DC, USA: National Academies Press; 2011.
-
- Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–462. - PubMed
-
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. - PubMed
-
- Leadley RM, Armstrong N, Lee YC, Allen A, Kleijnen J. Chronic diseases in the European Union: the prevalence and health cost implications of chronic pain. J Pain Palliat Care Pharmacother. 2012;26(4):310–325. - PubMed
-
- Ead H. Improving pain management for critically ill and injured patients. Dynamics. 2005;16(3):26–31. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

