Non-carrier nanoparticles adjuvant modular protein vaccine in a particle-dependent manner
- PMID: 25756283
- PMCID: PMC4355484
- DOI: 10.1371/journal.pone.0117203
Non-carrier nanoparticles adjuvant modular protein vaccine in a particle-dependent manner
Abstract
Nanoparticles are increasingly used to adjuvant vaccine formulations due to their biocompatibility, ease of manufacture and the opportunity to tailor their size, shape, and physicochemical properties. The efficacy of similarly-sized silica (Si-OH), poly (D,L-lactic-co-glycolic acid) (PLGA) and poly caprolactone (PCL) nanoparticles (nps) to adjuvant recombinant capsomere presenting antigenic M2e modular peptide from Influenza A virus (CapM2e) was investigated in vivo. Formulation of CapM2e with Si-OH or PLGA nps significantly boosted the immunogenicity of modular capsomeres, even though CapM2e was not actively attached to the nanoparticles prior to injection (i.e., formulation was by simple mixing). In contrast, PCL nps showed no significant adjuvant effect using this simple-mixing approach. The immune response induced by CapM2e alone or formulated with nps was antibody-biased with very high antigen-specific antibody titer and less than 20 cells per million splenocytes secreting interferon gamma. Modification of silica nanoparticle surface properties through amine functionalization and pegylation did not lead to significant changes in immune response. This study confirms that simple mixing-based formulation can lead to effective adjuvanting of antigenic protein, though with antibody titer dependent on nanoparticle physicochemical properties.
Conflict of interest statement
Figures
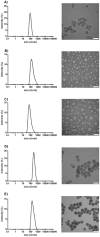
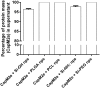
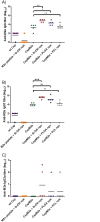

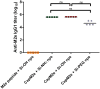
Similar articles
-
Entrapment of H1N1 Influenza Virus Derived Conserved Peptides in PLGA Nanoparticles Enhances T Cell Response and Vaccine Efficacy in Pigs.PLoS One. 2016 Apr 19;11(4):e0151922. doi: 10.1371/journal.pone.0151922. eCollection 2016. PLoS One. 2016. Retraction in: PLoS One. 2024 Jul 16;19(7):e0307483. doi: 10.1371/journal.pone.0307483. PMID: 27093541 Free PMC article. Retracted.
-
Co-administration of non-carrier nanoparticles boosts antigen immune response without requiring protein conjugation.Vaccine. 2014 Jun 17;32(29):3664-9. doi: 10.1016/j.vaccine.2014.04.043. Epub 2014 Apr 29. Vaccine. 2014. PMID: 24793947
-
Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: effect of mucoadhesive coating on antigen uptake and immune adjuvant activity.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):550-9. doi: 10.1016/j.ejpb.2013.06.017. Epub 2013 Jul 4. Eur J Pharm Biopharm. 2013. PMID: 23831265
-
Innovative Vaccine Strategy: Self-Adjuvanting Conjugate Vaccines.Methods Mol Biol. 2023;2613:55-72. doi: 10.1007/978-1-0716-2910-9_5. Methods Mol Biol. 2023. PMID: 36587070 Review.
-
Iron nanoparticles as novel vaccine adjuvants.Eur J Pharm Sci. 2021 Apr 1;159:105718. doi: 10.1016/j.ejps.2021.105718. Epub 2021 Jan 16. Eur J Pharm Sci. 2021. PMID: 33465476 Review.
Cited by
-
Nano-Microparticle Platforms in Developing Next-Generation Vaccines.Vaccines (Basel). 2021 Jun 5;9(6):606. doi: 10.3390/vaccines9060606. Vaccines (Basel). 2021. PMID: 34198865 Free PMC article. Review.
-
Layered double hydroxide nanoparticles as an adjuvant for inactivated foot-and-mouth disease vaccine in pigs.BMC Vet Res. 2020 Dec 4;16(1):474. doi: 10.1186/s12917-020-02689-6. BMC Vet Res. 2020. PMID: 33276787 Free PMC article.
-
Effect of Chitosan and Liposome Nanoparticles as Adjuvant Codelivery on the Immunoglobulin G Subclass Distribution in a Mouse Model.J Immunol Res. 2017;2017:9125048. doi: 10.1155/2017/9125048. Epub 2017 Jul 5. J Immunol Res. 2017. PMID: 28758135 Free PMC article.
-
Why have nanotechnologies been underutilized in the global uprising against the coronavirus pandemic?Nanomedicine (Lond). 2020 Jul;15(17):1719-1734. doi: 10.2217/nnm-2020-0163. Epub 2020 May 28. Nanomedicine (Lond). 2020. PMID: 32462968 Free PMC article. Review.
-
Alternative Methods of Vaccine Delivery: An Overview of Edible and Intradermal Vaccines.J Immunol Res. 2019 Mar 4;2019:8303648. doi: 10.1155/2019/8303648. eCollection 2019. J Immunol Res. 2019. PMID: 30949518 Free PMC article. Review.
References
-
- Plotkin SL, Plotkin SA (2004) A short history of vaccination. Vaccines 5: 1–16.
-
- Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, et al. (2006) Pathogen recognition and development of particulate vaccines: does size matter? Methods 40: 1–9. - PubMed
-
- Singh M, Chakrapani A, O’Hagan D (2007) Nanoparticles and microparticles as vaccine-delivery systems. Expert review of vaccines 6: 797–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

