Salmonellae interactions with host processes
- PMID: 25749450
- PMCID: PMC5074537
- DOI: 10.1038/nrmicro3420
Salmonellae interactions with host processes
Abstract
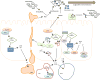
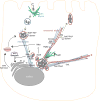
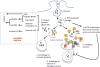
Salmonellae invasion and intracellular replication within host cells result in a range of diseases, including gastroenteritis, bacteraemia, enteric fever and focal infections. In recent years, considerable progress has been made in our understanding of the molecular mechanisms that salmonellae use to alter host cell physiology; through the delivery of effector proteins with specific activities and through the modulation of defence and stress response pathways. In this Review, we summarize our current knowledge of the complex interplay between bacterial and host factors that leads to inflammation, disease and, in most cases, control of the infection by its animal hosts, with a particular focus on Salmonella enterica subsp. enterica serovar Typhimurium. We also highlight gaps in our knowledge of the contributions of salmonellae and the host to disease pathogenesis, and we suggest future avenues for further study.
Figures

Similar articles
-
Salmonellae interplay with host cells.Nat Rev Microbiol. 2008 Jan;6(1):53-66. doi: 10.1038/nrmicro1788. Nat Rev Microbiol. 2008. PMID: 18026123 Review.
-
Salmonella Typhimurium and inflammation: a pathogen-centric affair.Nat Rev Microbiol. 2021 Nov;19(11):716-725. doi: 10.1038/s41579-021-00561-4. Epub 2021 May 19. Nat Rev Microbiol. 2021. PMID: 34012042 Free PMC article. Review.
-
Salmonella interactions with host cells: in vitro to in vivo.Philos Trans R Soc Lond B Biol Sci. 2000 May 29;355(1397):623-31. doi: 10.1098/rstb.2000.0603. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10874735 Free PMC article. Review.
-
Innate immune response to Salmonella typhimurium, a model enteric pathogen.Gut Microbes. 2012 Mar-Apr;3(2):62-70. doi: 10.4161/gmic.19141. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22198618 Free PMC article. Review.
-
SopD acts cooperatively with SopB during Salmonella enterica serovar Typhimurium invasion.Cell Microbiol. 2007 Dec;9(12):2839-55. doi: 10.1111/j.1462-5822.2007.01000.x. Epub 2007 Aug 13. Cell Microbiol. 2007. PMID: 17696999
Cited by
-
The Salmonella Effector SseK3 Targets Small Rab GTPases.Front Cell Infect Microbiol. 2020 Aug 19;10:419. doi: 10.3389/fcimb.2020.00419. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974215 Free PMC article.
-
Genetic code expansion enables visualization of Salmonella type three secretion system components and secreted effectors.Elife. 2021 Jun 1;10:e67789. doi: 10.7554/eLife.67789. Elife. 2021. PMID: 34061032 Free PMC article.
-
A trafficome-wide RNAi screen reveals deployment of early and late secretory host proteins and the entire late endo-/lysosomal vesicle fusion machinery by intracellular Salmonella.PLoS Pathog. 2020 Jul 13;16(7):e1008220. doi: 10.1371/journal.ppat.1008220. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32658937 Free PMC article.
-
Campylobacter fetus Induced Proinflammatory Response in Bovine Endometrial Epithelial Cells.Pol J Microbiol. 2021 Mar;70(1):99-106. doi: 10.33073/pjm-2021-009. Epub 2021 Mar 19. Pol J Microbiol. 2021. PMID: 33815531 Free PMC article.
-
Isolation, Identification, and Genetic Characterization of Antibiotic Resistance of Salmonella Species Isolated from Chicken Farms.J Trop Med. 2022 Nov 29;2022:6065831. doi: 10.1155/2022/6065831. eCollection 2022. J Trop Med. 2022. PMID: 36482931 Free PMC article.
References
-
- Pegues DA, Ohl ME, Miller SI. In: Salmonella species, including Salmonella typhi. Mandell GL, Bennett JE, Dolin R, editors. Elsevier/Churchill Livingstone; New York: 2005.
-
- Harris JC, Dupont HL, Hornick RB. Fecal leukocytes in diarrheal illness. Ann Intern Med. 1972;76:697–703. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

