Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans
- PMID: 25748562
- PMCID: PMC4449284
- DOI: 10.1097/ADM.0000000000000118
Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans
Abstract
Objectives: Cannabidiol (CBD) is hypothesized as a potential treatment for opioid addiction, with safety studies an important first step for medication development. We determined CBD safety and pharmacokinetics when administered concomitantly with a high-potency opioid in healthy subjects.
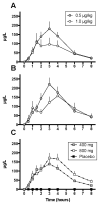
Methods: This double-blind, placebo-controlled cross-over study of CBD, coadministered with intravenous fentanyl, was conducted at the Clinical Research Center in Mount Sinai Hospital, a tertiary care medical center in New York City. Participants were healthy volunteers aged 21 to 65 years with prior opioid exposure, regardless of the route. Blood samples were obtained before and after 400 or 800 mg of CBD pretreatment, followed by a single 0.5 (session 1) or 1.0 μg/kg (session 2) of intravenous fentanyl dose. The primary outcome was the Systematic Assessment for Treatment Emergent Events (SAFTEE) to assess safety and adverse effects. CBD peak plasma concentrations, time to reach peak plasma concentrations (tmax), and area under the curve (AUC) were measured.
Results: SAFTEE data were similar between groups without respiratory depression or cardiovascular complications during any test session. After low-dose CBD, tmax occurred at 3 and 1.5 hours in sessions 1 and 2, respectively. After high-dose CBD, tmax occurred at 3 and 4 hours in sessions 1 and 2, respectively. There were no significant differences in plasma CBD or cortisol (AUC P = NS) between sessions.
Conclusions: Cannabidiol does not exacerbate adverse effects associated with intravenous fentanyl administration. Coadministration of CBD and opioids was safe and well tolerated. These data provide the foundation for future studies examining CBD as a potential treatment for opioid abuse.
Conflict of interest statement
Figures

Similar articles
-
A Phase 1, Randomised, Placebo-Controlled, Dose Escalation Study to Investigate the Safety, Tolerability and Pharmacokinetics of Cannabidiol in Fed Healthy Volunteers.Eur J Drug Metab Pharmacokinet. 2020 Oct;45(5):575-586. doi: 10.1007/s13318-020-00624-6. Eur J Drug Metab Pharmacokinet. 2020. PMID: 32409982 Free PMC article. Clinical Trial.
-
A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects.CNS Drugs. 2018 Nov;32(11):1053-1067. doi: 10.1007/s40263-018-0578-5. CNS Drugs. 2018. PMID: 30374683 Free PMC article. Clinical Trial.
-
Relative bioavailability of the fentanyl effervescent buccal tablet (FEBT) 1,080 pg versus oral transmucosal fentanyl citrate 1,600 pg and dose proportionality of FEBT 270 to 1,300 microg: a single-dose, randomized, open-label, three-period study in healthy adult volunteers.Clin Ther. 2006 May;28(5):715-24. doi: 10.1016/j.clinthera.2006.05.016. Clin Ther. 2006. PMID: 16861093 Clinical Trial.
-
Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers.Curr Pharm Des. 2012;18(32):4966-79. doi: 10.2174/138161212802884780. Curr Pharm Des. 2012. PMID: 22716148 Review.
-
Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone).Pain Pract. 2008 Jul-Aug;8(4):287-313. doi: 10.1111/j.1533-2500.2008.00204.x. Epub 2008 May 23. Pain Pract. 2008. PMID: 18503626
Cited by
-
Comparison of Five Oral Cannabidiol Preparations in Adult Humans: Pharmacokinetics, Body Composition, and Heart Rate Variability.Pharmaceuticals (Basel). 2021 Jan 6;14(1):35. doi: 10.3390/ph14010035. Pharmaceuticals (Basel). 2021. PMID: 33418866 Free PMC article.
-
New Approaches in Drug Dependence: Opioids.Curr Addict Rep. 2021;8(2):298-305. doi: 10.1007/s40429-021-00373-9. Epub 2021 May 26. Curr Addict Rep. 2021. PMID: 34055568 Free PMC article. Review.
-
A New Data Repository for Pharmacokinetic Natural Product-Drug Interactions: From Chemical Characterization to Clinical Studies.Drug Metab Dispos. 2020 Oct;48(10):1104-1112. doi: 10.1124/dmd.120.000054. Epub 2020 Jun 29. Drug Metab Dispos. 2020. PMID: 32601103 Free PMC article.
-
The Effectiveness and Safety of Cannabidiol in Non-seizure-related Indications: A Systematic Review of Published Randomized Clinical Trials.Pharmaceut Med. 2022 Dec;36(6):353-385. doi: 10.1007/s40290-022-00446-8. Epub 2022 Oct 21. Pharmaceut Med. 2022. PMID: 36271316 Free PMC article.
-
Efficacy of cannabinoids compared to the current standard treatments on symptom relief in persons with multiple sclerosis (CANSEP trial): study protocol for a randomized clinical trial.Front Neurol. 2024 Jul 24;15:1440678. doi: 10.3389/fneur.2024.1440678. eCollection 2024. Front Neurol. 2024. PMID: 39114536 Free PMC article.
References
-
- Agurell S, Carlsson S, Lindgren JE, et al. Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia. 1981;37:1090–2. - PubMed
-
- Agurell S, Halldin M, Lindgren JE, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–43. - PubMed
-
- Barta WD, Kurth ME, Stein MD, et al. Craving and self-efficacy in the first five weeks of methadone maintenance therapy: a daily process study. J Stud Alcohol Drugs. 2009;70:735–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

