Correlation of extended RAS and PIK3CA gene mutation status with outcomes from the phase III AGITG MAX STUDY involving capecitabine alone or in combination with bevacizumab plus or minus mitomycin C in advanced colorectal cancer
- PMID: 25742472
- PMCID: PMC4366893
- DOI: 10.1038/bjc.2015.37
Correlation of extended RAS and PIK3CA gene mutation status with outcomes from the phase III AGITG MAX STUDY involving capecitabine alone or in combination with bevacizumab plus or minus mitomycin C in advanced colorectal cancer
Abstract
Background: Mutations affecting RAS genes are now established predictive markers of nonresponse to anti-EGFR antibodies in advanced CRC. This analysis assessed the prognostic and predictive impact of extended RAS and PIK3CA gene mutation status in patients receiving capecitabine plus or minus bevacizumab (±mitomycin C) in the randomised phase III MAX study.
Methods: DNA was extracted from archival macrodissected formalin-fixed paraffin-embedded tumour tissue. Mutation status was determined using pyrosequencing, confirmed with Sanger sequencing (for equivocal RAS) and correlated with efficacy outcomes. Predictive analyses were undertaken using a test for interaction involving both C vs CB+CBM.
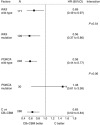
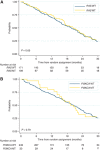
Results: Of the available 280 of the 471 (59.4%) patients, mutations in KRAS exons 2, 3 and 4 and NRAS 2, 3 and 4 were as follows: 32%, 2.9%, 2.2%, 1.4%, 0.7% and 0% (total RAS MT 39%). The PIK3CA MT rate was 7.5% exon 9 and 3.6% exon 20. Extended RAS gene mutation status (WT vs MT) had no prognostic impact for PFS (HR 0.91 (0.71-1.17)) or OS (HR 0.95 (0.71-1.25)). The RAS gene mutation status was not predictive of the effectiveness of bevacizumab for PFS (HR 0.56 (0.37-0.85) for RAS MT and HR 0.69 (0.5-0.97) for RAS WT; P for interaction 0.50). The PIK3CA mutation was neither predictive for bevacizumab effect nor prognostic.
Conclusion: Of KRAS exon 2 WT patients, 10% had additional RAS mutations. Neither all RAS gene mutation status nor PIK3CA mutation status was prognostic for PFS or OS, or predictive of bevacizumab outcome in patients with advanced CRC.
Figures
Similar articles
-
Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer.J Clin Oncol. 2011 Jul 1;29(19):2675-82. doi: 10.1200/JCO.2010.34.5520. Epub 2011 Jun 6. J Clin Oncol. 2011. PMID: 21646616 Clinical Trial.
-
Prognostic impact and the relevance of PTEN copy number alterations in patients with advanced colorectal cancer (CRC) receiving bevacizumab.Cancer Med. 2013 Jun;2(3):277-85. doi: 10.1002/cam4.75. Epub 2013 Mar 25. Cancer Med. 2013. PMID: 23930204 Free PMC article. Clinical Trial.
-
Role of Kras status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab: a TTD group cooperative study.PLoS One. 2012;7(10):e47345. doi: 10.1371/journal.pone.0047345. Epub 2012 Oct 12. PLoS One. 2012. PMID: 23174912 Free PMC article. Clinical Trial.
-
The role of antiangiogenic agents in the treatment of patients with advanced colorectal cancer according to K-RAS status.Angiogenesis. 2014 Oct;17(4):805-21. doi: 10.1007/s10456-014-9433-6. Epub 2014 May 3. Angiogenesis. 2014. PMID: 24793846 Review.
-
The prevalent KRAS exon 2 c.35 G>A mutation in metastatic colorectal cancer patients: A biomarker of worse prognosis and potential benefit of bevacizumab-containing intensive regimens?Crit Rev Oncol Hematol. 2015 Mar;93(3):190-202. doi: 10.1016/j.critrevonc.2014.10.004. Epub 2014 Oct 16. Crit Rev Oncol Hematol. 2015. PMID: 25459669 Review.
Cited by
-
Mutations in KRAS codon 12 predict poor survival in Chinese patients with metastatic colorectal cancer.Oncol Lett. 2018 Mar;15(3):3161-3166. doi: 10.3892/ol.2017.7709. Epub 2017 Dec 28. Oncol Lett. 2018. PMID: 29435051 Free PMC article.
-
PIK3CA and TP53 mutations predict overall survival of stage II/III colorectal cancer patients.World J Gastroenterol. 2018 Feb 7;24(5):631-640. doi: 10.3748/wjg.v24.i5.631. World J Gastroenterol. 2018. PMID: 29434452 Free PMC article.
-
K-RAS and N-RAS mutations in testicular germ cell tumors.Bosn J Basic Med Sci. 2017 May 20;17(2):159-163. doi: 10.17305/bjbms.2017.1764. Bosn J Basic Med Sci. 2017. PMID: 28426398 Free PMC article.
-
Current treatment options in RAS mutant metastatic colorectal cancer patients: a meta-analysis of 14 randomized phase III trials.J Cancer Res Clin Oncol. 2020 Aug;146(8):2077-2087. doi: 10.1007/s00432-020-03290-y. Epub 2020 Jun 19. J Cancer Res Clin Oncol. 2020. PMID: 32561975 Free PMC article. Review.
-
Immune Checkpoint Inhibitors in pMMR Metastatic Colorectal Cancer: A Tough Challenge.Cancers (Basel). 2020 Aug 17;12(8):2317. doi: 10.3390/cancers12082317. Cancers (Basel). 2020. PMID: 32824490 Free PMC article. Review.
References
-
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26 (10:1626–1634. - PubMed
-
- Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22 (7:1535–1546. - PubMed
-
- Bokemeyer C, Kohne C-H, Ciardiello F, Lenz H-J, Heinemann V, Klinkhardt U, Beier F, Duecker K, Tejpar S. Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab. J Clin Oncol. 2014;32 (Suppl 5:abstr 3505.
-
- Ciardiello F, Lenz H-J, Kohne C-H, Heinemann V, Tejpar S, Melezinek I, Beier F, Stroh C, Van Cutsem E. Treatment outcome according to tumor RAS mutation status in CRYSTAL study patients with metastatic colorectal cancer (mCRC) randomized to FOLFIRI with/without cetuximab. J Clin Oncol. 2014;32 (Suppl 5:abstr 3506.
-
- Ciardiello F, Troiani T, Bianco R, Orditura M, Morgillo F, Martinelli E, Morelli MP, Cascone T, Tortora G. Interaction between the epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor (VEGF) pathways: a rational approach for multi-target anticancer therapy. Ann Oncol. 2006;17 (Suppl 7:vii109–vii114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

