The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism
- PMID: 25733915
- PMCID: PMC4327407
- DOI: 10.4137/IJTR.S19985
The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism
Abstract
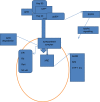
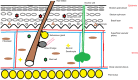
The aryl hydrocarbon receptor (AHR) is a cytosolic receptor for low molecular weight molecules, of which the most widely recognized ligand is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and the most widely recognized effect, chloracne. Adverse effects of manipulation were most recently and graphically demonstrated by the poisoning of Viktor Yushchenko during the Ukrainian presidential elections of 2004. However, recent research has revealed a receptor with wide-ranging, and at times, paradoxical actions. It was arguably among the first biological receptors to be utilized by dermatologists, dating from the time of topical tar preparations as a therapeutic agent. I provide a review outlining the role AHR plays in the development, cellular oxidation/antioxidation, responses to ultraviolet light, melanogenesis, epidermal barrier function, and immune regulation and its relationship to tryptophan metabolism. Finally, I will review the role of AHR in diseases of the integument.
Keywords: Aryl hydrocarbon receptor; UV exposure; cellular oxidation/antioxidation; epidermal barrier; immune regulation; melanogenesis; tryptophan metabolism.
Figures


Similar articles
-
AhR signalling and dioxin toxicity.Toxicol Lett. 2014 Oct 15;230(2):225-33. doi: 10.1016/j.toxlet.2013.10.039. Epub 2013 Nov 12. Toxicol Lett. 2014. PMID: 24239782 Review.
-
The tryptophan derivative 6-formylindolo[3,2-b]carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation.Crit Rev Toxicol. 2018 Aug;48(7):555-574. doi: 10.1080/10408444.2018.1493086. Epub 2018 Sep 18. Crit Rev Toxicol. 2018. PMID: 30226107 Review.
-
The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis.Pigment Cell Melanoma Res. 2010 Dec;23(6):828-33. doi: 10.1111/j.1755-148X.2010.00762.x. Pigment Cell Melanoma Res. 2010. PMID: 20973933
-
Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome.Toxicol Appl Pharmacol. 2016 Aug 1;304:121-32. doi: 10.1016/j.taap.2016.04.005. Epub 2016 Apr 20. Toxicol Appl Pharmacol. 2016. PMID: 27105554 Free PMC article.
-
Influence of light on aryl hydrocarbon receptor signaling and consequences in drug metabolism, physiology and disease.Expert Opin Drug Metab Toxicol. 2011 Oct;7(10):1267-93. doi: 10.1517/17425255.2011.614947. Epub 2011 Sep 2. Expert Opin Drug Metab Toxicol. 2011. PMID: 21883026 Review.
Cited by
-
Light Sensing beyond Vision: Focusing on a Possible Role for the FICZ/AhR Complex in Skin Optotransduction.Cells. 2024 Jun 22;13(13):1082. doi: 10.3390/cells13131082. Cells. 2024. PMID: 38994936 Free PMC article. Review.
-
Kynurenic Acid/AhR Signaling at the Junction of Inflammation and Cardiovascular Diseases.Int J Mol Sci. 2024 Jun 25;25(13):6933. doi: 10.3390/ijms25136933. Int J Mol Sci. 2024. PMID: 39000041 Free PMC article. Review.
-
PM2.5, Fine Particulate Matter: A Novel Player in the Epithelial-Mesenchymal Transition?Front Physiol. 2019 Nov 29;10:1404. doi: 10.3389/fphys.2019.01404. eCollection 2019. Front Physiol. 2019. PMID: 31849690 Free PMC article. Review.
-
Suppression of aberrant choroidal neovascularization through activation of the aryl hydrocarbon receptor.Biochim Biophys Acta Mol Basis Dis. 2018 May;1864(5 Pt A):1583-1595. doi: 10.1016/j.bbadis.2018.02.015. Epub 2018 Feb 23. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29481912 Free PMC article.
-
Is the Exposome Involved in Brain Disorders through the Serotoninergic System?Biomedicines. 2021 Sep 29;9(10):1351. doi: 10.3390/biomedicines9101351. Biomedicines. 2021. PMID: 34680468 Free PMC article. Review.
References
-
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43(1):309–34. - PubMed
-
- Carlstedt-Duke JM. Tissue distribution of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. Cancer Res. 1979;39(8):3172–6. - PubMed
-
- Johansson G, Gillner M, Högberg B, Gustafsson JA. The TCDD receptor in rat intestinal mucosa and its possible dietary ligands. Nutr Cancer. 1981;3(3):134–44. - PubMed
-
- Agostinis P, Garmyn M, Van Laethem A. The Aryl hydrocarbon receptor: an illuminating effector of the UVB response. Sci Signal. 2007;2007(403):e49. - PubMed
-
- Enan E, Matsumura F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem Pharmacol. 1996;52(10):1599–612. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

