Adhesion in mammary development: novel roles for E-cadherin in individual and collective cell migration
- PMID: 25733146
- PMCID: PMC4696070
- DOI: 10.1016/bs.ctdb.2014.12.001
Adhesion in mammary development: novel roles for E-cadherin in individual and collective cell migration
Abstract
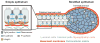
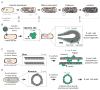
Epithelial tissues are essential for barrier function, secretion, and regulation of fluid transport. Their function requires cell polarity and cell-cell adhesion, mediated through intercellular junctions. Conversely, disruption of adhesion and polarity is thought to drive cancer progression. The mammary gland is an important model for cell adhesion due to its postnatal hormonally regulated development; ducts undergo branching morphogenesis in response to steroid hormones during puberty. These hormonal signals induce a transition from simple to stratified architecture, initiated by asymmetric luminal cell divisions. Ductal elongation is accomplished by this multilayered, low-polarity epithelium, and polarity is reestablished as elongation ceases. The requirement for cell adhesion has been tested in 3D culture and in vivo, using gene deletion, knockdown, and misexpression in both developmental and homeostatic contexts. Attention has focused on E-cadherin, the major classical cadherin in luminal epithelial cells. Classic studies revealed a requirement for E-cadherin during lactation, and E-cadherin loss is widely posited to promote metastasis. However, recent findings demonstrated a broader requirement for E-cadherin during branching morphogenesis and homeostasis and also, surprisingly, in epithelial dissemination. These studies suggest that long-standing models of the role of adhesion in epithelial biology need to be revisited. Advances in inducible gene expression and knockdown, CRISPR/Cas9 technology, and fluorescent labeling of genetically modified cells offer the opportunity to test the roles of diverse adhesion systems and to develop a mechanistic understanding of how cell adhesion regulates development and cancer.
Keywords: Branching morphogenesis; Breast cancer; Cadherin switch; E-cadherin; Epithelial–mesenchymal transition; Mammary epithelium; Metastasis; P-cadherin.
© 2015 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Inappropriate P-cadherin expression in the mouse mammary epithelium is compatible with normal mammary gland function.Differentiation. 2003 Aug;71(6):361-73. doi: 10.1046/j.1432-0436.2003.7106005.x. Differentiation. 2003. PMID: 12919105
-
Expression of the cytoplasmic domain of E-cadherin induces precocious mammary epithelial alveolar formation and affects cell polarity and cell-matrix integrity.Dev Biol. 1999 Dec 15;216(2):491-506. doi: 10.1006/dbio.1999.9517. Dev Biol. 1999. PMID: 10642788
-
Roles for E-cadherin cell surface regulation in cancer.Mol Biol Cell. 2016 Nov 1;27(21):3233-3244. doi: 10.1091/mbc.E16-01-0058. Epub 2016 Aug 31. Mol Biol Cell. 2016. PMID: 27582386 Free PMC article.
-
Cadherins and the mammary gland.J Cell Biochem. 2005 Jun 1;95(3):488-96. doi: 10.1002/jcb.20419. J Cell Biochem. 2005. PMID: 15838893 Review.
-
Cellular foundations of mammary tubulogenesis.Semin Cell Dev Biol. 2014 Jul;31:124-31. doi: 10.1016/j.semcdb.2014.04.019. Epub 2014 Apr 18. Semin Cell Dev Biol. 2014. PMID: 24747369 Free PMC article. Review.
Cited by
-
A Robust Mammary Organoid System to Model Lactation and Involution-like Processes.Bio Protoc. 2021 Apr 20;11(8):e3996. doi: 10.21769/BioProtoc.3996. eCollection 2021 Apr 20. Bio Protoc. 2021. PMID: 34124297 Free PMC article.
-
EMT in cancer.Nat Rev Cancer. 2018 Feb;18(2):128-134. doi: 10.1038/nrc.2017.118. Epub 2018 Jan 12. Nat Rev Cancer. 2018. PMID: 29326430
-
Loss of STING impairs lactogenic differentiation.Development. 2024 Oct 1;151(19):dev202998. doi: 10.1242/dev.202998. Epub 2024 Oct 14. Development. 2024. PMID: 39399905 Free PMC article.
-
Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses.J Extracell Vesicles. 2021 Mar;10(5):e12071. doi: 10.1002/jev2.12071. Epub 2021 Mar 10. J Extracell Vesicles. 2021. PMID: 33732416 Free PMC article.
-
In primary airway epithelial cells, the unjamming transition is distinct from the epithelial-to-mesenchymal transition.Nat Commun. 2020 Oct 7;11(1):5053. doi: 10.1038/s41467-020-18841-7. Nat Commun. 2020. PMID: 33028821 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

