Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses
- PMID: 25726868
- PMCID: PMC4452150
- DOI: 10.1111/cas.12650
Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses
Abstract
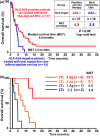
Recent genome-wide cDNA microarray analysis of gene expression profiles in comprehensive tumor types coupled with isolation of cancer tissues by laser-microbeam microdissection have revealed ideal tumor-associated antigens (TAAs) that are frequently overexpressed in various cancers including head and neck squamous cell cancer (HNSCC) and lung cancer, but not in most normal tissues except for testis, placenta, and fetal organs. Preclinical studies using HLA-transgenic mice and human T cells in vitro showed that TAA-derived CTL-epitope short peptides (SPs) are highly immunogenic and induce HLA-A2 or -A24-restricted CTLs. Based on the accumulated evidence, we carried out a phase II clinical trial of the TAA-SP vaccine in advanced 37 HNSCC patients. This study showed a significant induction of TAA-specific CTLs in the majority of patients without serious adverse effects. Importantly, clinical responses including a complete response were observed in this study. Another phase II clinical trial of therapeutic TAA-SP vaccine, designed to evaluate the ability of prevention of recurrence, is ongoing in HNSCC patients who have received curative operations. Further studies in human preclinical studies and in vivo studies using HLA class I transgenic mice showed TAA-derived long peptides (TAA-LPs) have the capacity to induce not only promiscuous HLA class II-restricted CD4(+) T helper type 1 cells but also tumor-specific CTLs through a cross-presentation mechanism. Moreover, we observed an augmentation of TAA-LP-specific T helper type 1 cell responses and tumor antigen-spreading in HNSCC patients vaccinated with TAA-SPs. This accumulated evidence suggests that therapeutic TAA-SPs and LPs vaccines may provide a promising cancer immunotherapy.
Keywords: CTL epitope; Cancer immunotherapy; cDNA microarray analysis; helper T-cell epitope; tumor-associated antigen.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures



Similar articles
-
Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T-cell epitopes: KIF20A-specific CD4+ T-cell immunity in patients with malignant tumor.Clin Cancer Res. 2013 Aug 15;19(16):4508-20. doi: 10.1158/1078-0432.CCR-13-0197. Epub 2013 May 28. Clin Cancer Res. 2013. PMID: 23714729
-
Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients.Int J Cancer. 2014 Jan 15;134(2):352-66. doi: 10.1002/ijc.28376. Int J Cancer. 2014. PMID: 24734272
-
Identification of a novel tumor-associated antigen, cadherin 3/P-cadherin, as a possible target for immunotherapy of pancreatic, gastric, and colorectal cancers.Clin Cancer Res. 2008 Oct 15;14(20):6487-95. doi: 10.1158/1078-0432.CCR-08-1086. Clin Cancer Res. 2008. PMID: 18927288
-
Tecemotide: an antigen-specific cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3383-93. doi: 10.4161/hv.29836. Hum Vaccin Immunother. 2014. PMID: 25483673 Free PMC article. Review.
-
The present status and future prospects of peptide-based cancer vaccines.Int Immunol. 2016 Jul;28(7):319-28. doi: 10.1093/intimm/dxw027. Epub 2016 May 28. Int Immunol. 2016. PMID: 27235694 Review.
Cited by
-
Lung cancer in never smokers: Tumor immunology and challenges for immunotherapy.Front Immunol. 2022 Aug 24;13:984349. doi: 10.3389/fimmu.2022.984349. eCollection 2022. Front Immunol. 2022. PMID: 36091058 Free PMC article. Review.
-
From the era of ineffective tumor vaccines to a future with effective immunotherapy.J Thorac Dis. 2016 Aug;8(8):1916-7. doi: 10.21037/jtd.2016.07.04. J Thorac Dis. 2016. PMID: 27621843 Free PMC article. No abstract available.
-
Assessment of associations between clinical and immune microenvironmental factors and tumor mutation burden in resected nonsmall cell lung cancer by applying machine learning to whole-slide images.Cancer Med. 2020 Jul;9(13):4864-4875. doi: 10.1002/cam4.3107. Epub 2020 May 12. Cancer Med. 2020. PMID: 32400056 Free PMC article.
-
Proteomics-inspired precision medicine for treating and understanding multiple myeloma.Expert Rev Precis Med Drug Dev. 2020;5(2):67-85. doi: 10.1080/23808993.2020.1732205. Epub 2020 Feb 24. Expert Rev Precis Med Drug Dev. 2020. PMID: 34414281 Free PMC article.
-
Telomerase-Targeted Cancer Immunotherapy.Int J Mol Sci. 2019 Apr 12;20(8):1823. doi: 10.3390/ijms20081823. Int J Mol Sci. 2019. PMID: 31013796 Free PMC article. Review.
References
-
- Couzin-Frankel J. Breakthrough of the year “Cancer Immunotherapy”. Science. 2013;342:1432–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

