Regulation of inflammation and fibrosis by macrophages in lymphedema
- PMID: 25724493
- PMCID: PMC4551121
- DOI: 10.1152/ajpheart.00598.2014
Regulation of inflammation and fibrosis by macrophages in lymphedema
Abstract
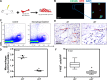
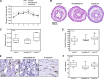
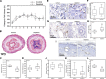
Lymphedema, a common complication of cancer treatment, is characterized by inflammation, fibrosis, and adipose deposition. We have previously shown that macrophage infiltration is increased in mouse models of lymphedema. Because macrophages are regulators of lymphangiogenesis and fibrosis, this study aimed to determine the role of these cells in lymphedema using depletion experiments. Matched biopsy specimens of normal and lymphedema tissues were obtained from patients with unilateral upper extremity breast cancer-related lymphedema, and macrophage accumulation was assessed using immunohistochemistry. In addition, we used a mouse tail model of lymphedema to quantify macrophage accumulation and analyze outcomes of conditional macrophage depletion. Histological analysis of clinical lymphedema biopsies revealed significantly increased macrophage infiltration. Similarly, in the mouse tail model, lymphatic injury increased the number of macrophages and favored M2 differentiation. Chronic macrophage depletion using lethally irradiated wild-type mice reconstituted with CD11b-diphtheria toxin receptor mouse bone marrow did not decrease swelling, adipose deposition, or overall inflammation. Macrophage depletion after lymphedema had become established significantly increased fibrosis and accumulation of CD4(+) cells and promoted Th2 differentiation while decreasing lymphatic transport capacity and VEGF-C expression. Our findings suggest that macrophages home to lymphedematous tissues and differentiate into the M2 phenotype. In addition, our findings suggest that macrophages have an antifibrotic role in lymphedema and either directly or indirectly regulate CD4(+) cell accumulation and Th2 differentiation. Finally, our findings suggest that lymphedema-associated macrophages are a major source of VEGF-C and that impaired macrophage responses after lymphatic injury result in decreased lymphatic function.
Keywords: diphtheria toxin; fibrosis; inflammation; lymphatic function; lymphedema; macrophages.
Copyright © 2015 the American Physiological Society.
Figures





Similar articles
-
Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury.Am J Physiol Cell Physiol. 2012 Feb 15;302(4):C709-19. doi: 10.1152/ajpcell.00284.2011. Epub 2011 Nov 2. Am J Physiol Cell Physiol. 2012. PMID: 22049214 Free PMC article.
-
Increased interstitial protein because of impaired lymph drainage does not induce fibrosis and inflammation in lymphedema.Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):266-74. doi: 10.1161/ATVBAHA.112.300384. Epub 2013 Jan 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23288156
-
Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair.Am J Pathol. 2010 Dec;177(6):3202-14. doi: 10.2353/ajpath.2010.100594. Epub 2010 Nov 5. Am J Pathol. 2010. PMID: 21056998 Free PMC article.
-
Regulation of Immune Function by the Lymphatic System in Lymphedema.Front Immunol. 2019 Mar 18;10:470. doi: 10.3389/fimmu.2019.00470. eCollection 2019. Front Immunol. 2019. PMID: 30936872 Free PMC article. Review.
-
Modulation of Immunity by Lymphatic Dysfunction in Lymphedema.Front Immunol. 2019 Jan 29;10:76. doi: 10.3389/fimmu.2019.00076. eCollection 2019. Front Immunol. 2019. PMID: 30761143 Free PMC article. Review.
Cited by
-
Dietary supplements in lymphedema.J Prev Med Hyg. 2022 Oct 17;63(2 Suppl 3):E200-E205. doi: 10.15167/2421-4248/jpmh2022.63.2S3.2761. eCollection 2022 Jun. J Prev Med Hyg. 2022. PMID: 36479479 Free PMC article. Review.
-
Fibrosis and secondary lymphedema: chicken or egg?Transl Res. 2019 Jul;209:68-76. doi: 10.1016/j.trsl.2019.04.001. Epub 2019 Apr 4. Transl Res. 2019. PMID: 31022376 Free PMC article. Review.
-
Lymphatic Vasculature in Energy Homeostasis and Obesity.Front Physiol. 2020 Jan 22;11:3. doi: 10.3389/fphys.2020.00003. eCollection 2020. Front Physiol. 2020. PMID: 32038308 Free PMC article. Review.
-
Characterization of Immune Cell Infiltration and Collagen Type III Disorganization in Human Secondary Lymphedema: A Case-control Study.Plast Reconstr Surg Glob Open. 2024 Jun 17;12(6):e5906. doi: 10.1097/GOX.0000000000005906. eCollection 2024 Jun. Plast Reconstr Surg Glob Open. 2024. PMID: 38911579 Free PMC article.
-
Characterizing early postoperative changes in body composition in patients with secondary lymphedema after breast cancer surgery: potential screening indicators for preventive intervention.J Phys Ther Sci. 2024 Oct;36(10):672-676. doi: 10.1589/jpts.36.672. Epub 2024 Oct 1. J Phys Ther Sci. 2024. PMID: 39354926 Free PMC article.
References
-
- Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295: H2113–H2127, 2008. - PubMed
-
- Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 116: 5138–5149, 2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

