Gammaherpesvirus Tegument Protein ORF33 Is Associated With Intranuclear Capsids at an Early Stage of the Tegumentation Process
- PMID: 25717105
- PMCID: PMC4442515
- DOI: 10.1128/JVI.00079-15
Gammaherpesvirus Tegument Protein ORF33 Is Associated With Intranuclear Capsids at an Early Stage of the Tegumentation Process
Abstract
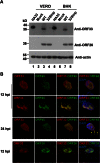
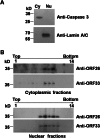
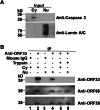
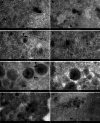
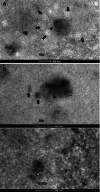
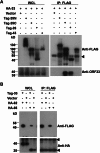
Herpesvirus nascent capsids, after assembly in the nucleus, must acquire a variety of tegument proteins during maturation. However, little is known about the identity of the tegument proteins that are associated with capsids in the nucleus or the molecular mechanisms involved in the nuclear egress of capsids into the cytoplasm, especially for the two human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), due to a lack of efficient lytic replication systems. Murine gammaherpesvirus 68 (MHV-68) is genetically related to human gammaherpesviruses and serves as an excellent model to study the de novo lytic replication of gammaherpesviruses. We have previously shown that open reading frame 33 (ORF33) of MHV-68 is a tegument protein of mature virions and is essential for virion assembly and egress. However, it remains unclear how ORF33 is incorporated into virions. In this study, we first show that the endogenous ORF33 protein colocalizes with capsid proteins at discrete areas in the nucleus during viral infection. Cosedimentation analysis as well as an immunoprecipitation assay demonstrated that ORF33 is associated with both nuclear and cytoplasmic capsids. An immunogold labeling experiment using an anti-ORF33 monoclonal antibody revealed that ORF33-rich areas in the nucleus are surrounded by immature capsids. Moreover, ORF33 is associated with nucleocapsids prior to primary envelopment as well as with mature virions in the cytoplasm. Finally, we show that ORF33 interacts with two capsid proteins, suggesting that nucleocapsids may interact with ORF33 in a direct manner. In summary, we identified ORF33 to be a tegument protein that is associated with intranuclear capsids prior to primary envelopment, likely through interacting with capsid proteins in a direct manner.
Importance: Morphogenesis is an essential step in virus propagation that leads to the generation of progeny virions. For herpesviruses, this is a complicated process that starts in the nucleus. Although the process of capsid assembly and genome packaging is relatively well understood, how capsids acquire tegument (the layer between the capsid and the envelope in a herpesvirus virion) and whether the initial tegumentation process takes place in the nucleus remain unclear. We previously showed that ORF33 of MHV-68 is a tegument protein and functions in both the nuclear egress of capsids and final virion maturation in the cytoplasm. In the present study, we show that ORF33 is associated with intranuclear capsids prior to primary envelopment and identify novel interactions between ORF33 and two capsid proteins. Our work provides new insights into the association between tegument proteins and nucleocapsids at an early stage of the virion maturation process for herpesviruses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
The Interaction between Tegument Proteins ORF33 and ORF45 Plays an Essential Role in Cytoplasmic Virion Maturation of a Gammaherpesvirus.J Virol. 2022 Nov 23;96(22):e0107322. doi: 10.1128/jvi.01073-22. Epub 2022 Oct 27. J Virol. 2022. PMID: 36300940 Free PMC article.
-
Open reading frame 33 of a gammaherpesvirus encodes a tegument protein essential for virion morphogenesis and egress.J Virol. 2009 Oct;83(20):10582-95. doi: 10.1128/JVI.00497-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656880 Free PMC article.
-
Murine gammaherpesvirus-68 ORF38 encodes a tegument protein and is packaged into virions during secondary envelopment.Protein Cell. 2014 Feb;5(2):141-50. doi: 10.1007/s13238-013-0005-0. Epub 2014 Jan 29. Protein Cell. 2014. PMID: 24474202 Free PMC article.
-
Nuclear Egress of Herpesviruses: The Prototypic Vesicular Nucleocytoplasmic Transport.Adv Virus Res. 2016;94:81-140. doi: 10.1016/bs.aivir.2015.10.002. Epub 2016 Jan 29. Adv Virus Res. 2016. PMID: 26997591 Review.
-
Comprehensive Analysis of the Tegument Proteins Involved in Capsid Transport and Virion Morphogenesis of Alpha, Beta and Gamma Herpesviruses.Viruses. 2023 Oct 6;15(10):2058. doi: 10.3390/v15102058. Viruses. 2023. PMID: 37896835 Free PMC article. Review.
Cited by
-
The Product of the Herpes Simplex Virus 2 UL16 Gene Is Critical for the Egress of Capsids from the Nuclei of Infected Cells.J Virol. 2017 Apr 28;91(10):e00350-17. doi: 10.1128/JVI.00350-17. Print 2017 May 15. J Virol. 2017. PMID: 28275195 Free PMC article.
-
Tegument Protein ORF45 Plays an Essential Role in Virion Morphogenesis of Murine Gammaherpesvirus 68.J Virol. 2016 Jul 27;90(16):7587-7592. doi: 10.1128/JVI.03231-15. Print 2016 Aug 15. J Virol. 2016. PMID: 27226376 Free PMC article.
-
Phosphorylation of Bovine Herpesvirus 1 VP8 Plays a Role in Viral DNA Encapsidation and Is Essential for Its Cytoplasmic Localization and Optimal Virion Incorporation.J Virol. 2016 Apr 14;90(9):4427-4440. doi: 10.1128/JVI.00219-16. Print 2016 May. J Virol. 2016. PMID: 26889039 Free PMC article.
-
Liquid-liquid phase separation mediates the formation of herpesvirus assembly compartments.J Cell Biol. 2023 Jan 2;222(1):e202201088. doi: 10.1083/jcb.202201088. Epub 2022 Oct 17. J Cell Biol. 2023. PMID: 36250941 Free PMC article.
-
Functional Identification and Characterization of the Nuclear Egress Complex of a Gammaherpesvirus.J Virol. 2019 Nov 26;93(24):e01422-19. doi: 10.1128/JVI.01422-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31554685 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

