Hippocampal Wnt Signaling: Memory Regulation and Hormone Interactions
- PMID: 25717070
- PMCID: PMC7053425
- DOI: 10.1177/1073858415574728
Hippocampal Wnt Signaling: Memory Regulation and Hormone Interactions
Abstract
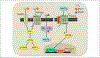
Wnt signaling has emerged in recent years as a major player in both nervous system development and adult synaptic plasticity. Of particular relevance to researchers studying learning and memory, Wnt signaling is critical for normal functioning of the hippocampus, a brain region that is essential for many types of memory formation and whose dysfunction is implicated in numerous neurodegenerative and psychiatric conditions. Impaired hippocampal Wnt signaling is implicated in several of these conditions, however, little is known about how Wnt signaling mediates hippocampal memory formation. This review will provide a general overview of Wnt signaling and discuss evidence demonstrating a key role for Wnt signaling in hippocampal memory formation in both normal and disease states. The regulation of Wnt signaling by ovarian sex steroid hormones will also be highlighted, given that the neuroprotection afforded by Wnt-hormone interactions may have significant implications for cognitive function in aging, neurodegenerative disease, and ischemic injury.
Keywords: Alzheimer’s disease; GSK3β; estradiol; hippocampus; progesterone; β-catenin.
© The Author(s) 2015.
Conflict of interest statement
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
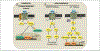
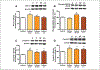
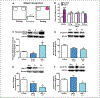
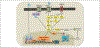
Similar articles
-
TGF-β1 Restores Hippocampal Synaptic Plasticity and Memory in Alzheimer Model via the PI3K/Akt/Wnt/β-Catenin Signaling Pathway.J Mol Neurosci. 2019 Jan;67(1):142-149. doi: 10.1007/s12031-018-1219-7. Epub 2018 Dec 12. J Mol Neurosci. 2019. PMID: 30539409
-
Role of Wnt signaling in synaptic plasticity and memory.Neurobiol Learn Mem. 2022 Jan;187:107558. doi: 10.1016/j.nlm.2021.107558. Epub 2021 Nov 19. Neurobiol Learn Mem. 2022. PMID: 34808336 Review.
-
Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer's disease.J Neural Transm (Vienna). 2015 Sep;122(9):1303-18. doi: 10.1007/s00702-015-1375-7. Epub 2015 Feb 14. J Neural Transm (Vienna). 2015. PMID: 25680440
-
Wnt signaling in the nervous system and in Alzheimer's disease.J Mol Cell Biol. 2014 Feb;6(1):64-74. doi: 10.1093/jmcb/mjt051. J Mol Cell Biol. 2014. PMID: 24549157 Review.
-
Resveratrol Mediated Regulation of Hippocampal Neuroregenerative Plasticity via SIRT1 Pathway in Synergy with Wnt Signaling: Neurotherapeutic Implications to Mitigate Memory Loss in Alzheimer's Disease.J Alzheimers Dis. 2023;94(s1):S125-S140. doi: 10.3233/JAD-220559. J Alzheimers Dis. 2023. PMID: 36463442 Free PMC article. Review.
Cited by
-
Sex differences in clinical cognitive impairment with Lewy bodies: a Chinese multicenter study.Biol Sex Differ. 2022 Oct 1;13(1):55. doi: 10.1186/s13293-022-00464-w. Biol Sex Differ. 2022. PMID: 36183142 Free PMC article.
-
Dickkopf-1 blocks 17β-estradiol-enhanced object memory consolidation in ovariectomized female mice.Horm Behav. 2019 Aug;114:104545. doi: 10.1016/j.yhbeh.2019.06.009. Epub 2019 Aug 12. Horm Behav. 2019. PMID: 31228421 Free PMC article.
-
Faecal microbiota transplantation, a promising way to treat colorectal cancer.EBioMedicine. 2019 Nov;49:13-14. doi: 10.1016/j.ebiom.2019.10.015. Epub 2019 Oct 21. EBioMedicine. 2019. PMID: 31648984 Free PMC article. No abstract available.
-
Inhibition of Soluble Epoxide Hydrolase Is Protective against the Multiomic Effects of a High Glycemic Diet on Brain Microvascular Inflammation and Cognitive Dysfunction.Nutrients. 2021 Nov 1;13(11):3913. doi: 10.3390/nu13113913. Nutrients. 2021. PMID: 34836168 Free PMC article.
-
Non-alcoholic Fatty Liver Disease: Also a Disease of the Brain? A Systematic Review of the Preclinical Evidence.Neurochem Res. 2024 Jun;49(6):1468-1488. doi: 10.1007/s11064-022-03551-x. Epub 2022 Mar 1. Neurochem Res. 2024. PMID: 35230646
References
-
- Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. 2004. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res 297:186–96. - PubMed
-
- Alzheimer’s Association. 2011. 2011 Alzheimer’s disease facts and figures. Washington, DC: Alzheimer’s Association; p 31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

