Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib
- PMID: 25700432
- PMCID: PMC4400288
- DOI: 10.1182/blood-2014-10-606038
Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib
Abstract
Ibrutinib is an orally administered inhibitor of Bruton tyrosine kinase that antagonizes B-cell receptor, chemokine, and integrin-mediated signaling. In early-phase studies, ibrutinib demonstrated high response rates and prolonged progression-free survival (PFS) in chronic lymphocytic leukemia (CLL). The durable responses observed with ibrutinib relate in part to a modest toxicity profile that allows the majority of patients to receive continuous therapy for an extended period. We report on median 3-year follow-up of 132 patients with symptomatic treatment-naïve and relapsed/refractory CLL or small lymphocytic lymphoma. Longer treatment with ibrutinib was associated with improvement in response quality over time and durable remissions. Toxicity with longer follow-up diminished with respect to occurrence of grade 3 or greater cytopenias, fatigue, and infections. Progression remains uncommon, occurring primarily in some patients with relapsed del(17)(p13.1) and/or del(11)(q22.3) disease. Treatment-related lymphocytosis remains largely asymptomatic even when persisting >1 year and does not appear to alter longer-term PFS and overall survival compared with patients with partial response or better. Collectively, these data provide evidence that ibrutinib controls CLL disease manifestations and is well tolerated for an extended period; this information can help direct potential treatment options for different subgroups to diminish the long-term risk of relapse.
© 2015 by The American Society of Hematology.
Figures
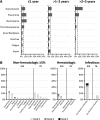
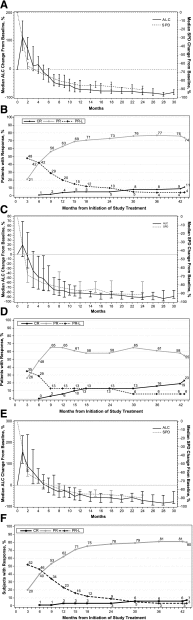
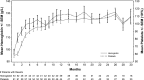


Comment in
-
Three years of ibrutinib in CLL.Blood. 2015 Apr 16;125(16):2455-6. doi: 10.1182/blood-2015-03-630772. Blood. 2015. PMID: 25883227
Similar articles
-
Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/II PCYC-1102 Study.Clin Cancer Res. 2020 Aug 1;26(15):3918-3927. doi: 10.1158/1078-0432.CCR-19-2856. Epub 2020 Mar 24. Clin Cancer Res. 2020. PMID: 32209572 Free PMC article. Clinical Trial.
-
Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma.Am J Hematol. 2019 Dec;94(12):1353-1363. doi: 10.1002/ajh.25638. Epub 2019 Oct 13. Am J Hematol. 2019. PMID: 31512258 Free PMC article. Clinical Trial.
-
Extended Treatment with Single-Agent Ibrutinib at the 420 mg Dose Leads to Durable Responses in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma.Clin Cancer Res. 2017 Mar 1;23(5):1149-1155. doi: 10.1158/1078-0432.CCR-16-1431. Epub 2017 Jan 10. Clin Cancer Res. 2017. PMID: 28073846 Free PMC article. Clinical Trial.
-
Ibrutinib for treatment of chronic lymphocytic leukemia.Am J Health Syst Pharm. 2016 Mar 15;73(6):367-75. doi: 10.2146/ajhp140760. Am J Health Syst Pharm. 2016. PMID: 26953281 Review.
-
Ibrutinib in CLL: a focus on adverse events, resistance, and novel approaches beyond ibrutinib.Ann Hematol. 2017 Jul;96(7):1175-1184. doi: 10.1007/s00277-017-2973-2. Epub 2017 Mar 24. Ann Hematol. 2017. PMID: 28342031 Review.
Cited by
-
Final 5-year findings from the phase 3 HELIOS study of ibrutinib plus bendamustine and rituximab in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma.Leuk Lymphoma. 2020 Dec;61(13):3188-3197. doi: 10.1080/10428194.2020.1795159. Epub 2020 Aug 6. Leuk Lymphoma. 2020. PMID: 32762271 Free PMC article. Clinical Trial.
-
Risk of Infectious Complications in Hemato-Oncological Patients Treated with Kinase Inhibitors.Biomark Insights. 2016 Apr 21;10(Suppl 3):55-68. doi: 10.4137/BMI.S22430. eCollection 2015. Biomark Insights. 2016. PMID: 27127405 Free PMC article. Review.
-
Unveiling the Cardiotoxicity Conundrum: Navigating the Seas of Tyrosine Kinase Inhibitor Therapies.Cancer Control. 2024 Jan-Dec;31:10732748241285755. doi: 10.1177/10732748241285755. Cancer Control. 2024. PMID: 39318033 Free PMC article. Review.
-
The Occurrence of Richter's Syndrome during Treatment with Obinutuzumab and Chlorambucil.Case Rep Hematol. 2020 Jul 16;2020:8363427. doi: 10.1155/2020/8363427. eCollection 2020. Case Rep Hematol. 2020. PMID: 32724682 Free PMC article.
-
Initial treatment of CLL: integrating biology and functional status.Blood. 2015 Jul 23;126(4):463-70. doi: 10.1182/blood-2015-04-585067. Epub 2015 Jun 11. Blood. 2015. PMID: 26065656 Free PMC article. Review.
References
-
- Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. - PMC - PubMed
-
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. - PubMed
-
- Lozanski G, Ruppert AS, Heerema NA, et al. Variations of the ataxia telangiectasia mutated gene in patients with chronic lymphocytic leukemia lack substantial impact on progression-free survival and overall survival: a Cancer and Leukemia Group B study. Leuk Lymphoma. 2012;53(9):1743–1748. - PMC - PubMed
-
- Austen B, Skowronska A, Baker C, et al. Mutation status of the residual ATM allele is an important determinant of the cellular response to chemotherapy and survival in patients with chronic lymphocytic leukemia containing an 11q deletion. J Clin Oncol. 2007;25(34):5448–5457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

