Regulation of postsynaptic function by the dementia-related ESCRT-III subunit CHMP2B
- PMID: 25698751
- PMCID: PMC4331633
- DOI: 10.1523/JNEUROSCI.0586-14.2015
Regulation of postsynaptic function by the dementia-related ESCRT-III subunit CHMP2B
Erratum in
- J Neurosci. 2015 May 20;35(20):8035-7
Abstract
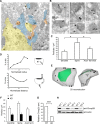
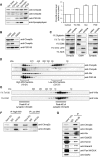
The charged multivesicular body proteins (Chmp1-7) are an evolutionarily conserved family of cytosolic proteins that transiently assembles into helical polymers that change the curvature of cellular membrane domains. Mutations in human CHMP2B cause frontotemporal dementia, suggesting that this protein may normally control some neuron-specific process. Here, we examined the function, localization, and interactions of neuronal Chmp2b. The protein was highly expressed in mouse brain and could be readily detected in neuronal dendrites and spines. Depletion of endogenous Chmp2b reduced dendritic branching of cultured hippocampal neurons, decreased excitatory synapse density in vitro and in vivo, and abolished activity-induced spine enlargement and synaptic potentiation. To understand the synaptic effects of Chmp2b, we determined its ultrastructural distribution by quantitative immuno-electron microscopy and its biochemical interactions by coimmunoprecipitation and mass spectrometry. In the hippocampus in situ, a subset of neuronal Chmp2b was shown to concentrate beneath the perisynaptic membrane of dendritic spines. In synaptoneurosome lysates, Chmp2b was stably bound to a large complex containing other members of the Chmp family, as well as postsynaptic scaffolds. The supramolecular Chmp assembly detected here corresponds to a stable form of the endosomal sorting complex required for transport-III (ESCRT-III), a ubiquitous cytoplasmic protein complex known to play a central role in remodeling of lipid membranes. We conclude that Chmp2b-containing ESCRT-III complexes are also present at dendritic spines, where they regulate synaptic plasticity. We propose that synaptic ESCRT-III filaments may function as a novel element of the submembrane cytoskeleton of spines.
Keywords: ESCRT filaments; frontotemporal dementia; postsynaptic scaffold; spinoskeleton; structural plasticity.
Copyright © 2015 Chassefeyre et al.
Figures


Similar articles
-
CHMP2B mutants linked to frontotemporal dementia impair maturation of dendritic spines.J Cell Sci. 2010 Sep 1;123(Pt 17):2943-54. doi: 10.1242/jcs.068817. Epub 2010 Aug 10. J Cell Sci. 2010. PMID: 20699355 Free PMC article.
-
A novel synaptopathy-defective synaptic vesicle protein trafficking in the mutant CHMP2B mouse model of frontotemporal dementia.J Neurochem. 2022 Feb;160(3):412-425. doi: 10.1111/jnc.15551. Epub 2021 Dec 11. J Neurochem. 2022. PMID: 34855215
-
A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis.J Neurosci. 2011 Apr 6;31(14):5414-25. doi: 10.1523/JNEUROSCI.2456-10.2011. J Neurosci. 2011. PMID: 21471377 Free PMC article.
-
The role of CHMP2BIntron5 in autophagy and frontotemporal dementia.Brain Res. 2016 Oct 15;1649(Pt B):151-157. doi: 10.1016/j.brainres.2016.02.051. Epub 2016 Mar 10. Brain Res. 2016. PMID: 26972529 Free PMC article. Review.
-
The role of ESCRT during development and functioning of the nervous system.Semin Cell Dev Biol. 2018 Feb;74:40-49. doi: 10.1016/j.semcdb.2017.08.013. Epub 2017 Aug 12. Semin Cell Dev Biol. 2018. PMID: 28811263 Review.
Cited by
-
Visualizing the native cellular organization by coupling cryofixation with expansion microscopy (Cryo-ExM).Nat Methods. 2022 Feb;19(2):216-222. doi: 10.1038/s41592-021-01356-4. Epub 2022 Jan 13. Nat Methods. 2022. PMID: 35027766 Free PMC article.
-
Expression of mutant CHMP2B linked to neurodegeneration in humans disrupts circadian rhythms in Drosophila.FASEB Bioadv. 2019 Jul 11;1(8):511-520. doi: 10.1096/fba.2019-00042. eCollection 2019 Aug. FASEB Bioadv. 2019. PMID: 32123847 Free PMC article.
-
Expression of a human variant of CHMP2B linked to neurodegeneration in Drosophila external sensory organs leads to cell fate transformations associated with increased Notch activity.Dev Neurobiol. 2020 Mar;80(3-4):85-97. doi: 10.1002/dneu.22722. Epub 2019 Oct 23. Dev Neurobiol. 2020. PMID: 31587468 Free PMC article.
-
Synaptic Interactome Mining Reveals p140Cap as a New Hub for PSD Proteins Involved in Psychiatric and Neurological Disorders.Front Mol Neurosci. 2017 Jun 30;10:212. doi: 10.3389/fnmol.2017.00212. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28713243 Free PMC article.
-
A two-tiered system for selective receptor and transporter protein degradation.PLoS Genet. 2022 Oct 10;18(10):e1010446. doi: 10.1371/journal.pgen.1010446. eCollection 2022 Oct. PLoS Genet. 2022. PMID: 36215320 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
