A comparative study of two different assay kits for the detection of secreted alkaline phosphatase in HPV antibody neutralization assays
- PMID: 25695397
- PMCID: PMC4514413
- DOI: 10.4161/21645515.2014.990851
A comparative study of two different assay kits for the detection of secreted alkaline phosphatase in HPV antibody neutralization assays
Abstract
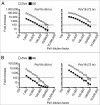
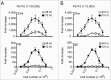
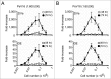
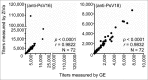
To assess immunogenicity and development of antibodies in the context of vaccination, it is critical to quantify titers of neutralizing antibodies. We have been employing the 293TT cell-based neutralization assay system to quantify anti-HPV neutralizing antibodies. In this system, human papillomavirus (HPV) pseudovirion (PsV) particles encapsidating secreted alkaline phosphatase (SEAP) gene are used to measure infection of 293TT cells in 72-hr cell-culture supernatants. SEAP has traditionally been measured by Great EscAPe™ SEAP Chemiluminescence Kit 2.0 (GE). To reduce the cost, and to potentially increase efficiency, we sought a cheaper kit with better detection capability. Performance characteristics of the newer chemiluminescence kit, ZiVa® Ultra SEAP Plus Assay (Ziva) and GE were compared using the 293TT system. Dose titration of HPV PsV 16 or 18 showed that signal-to-noise ratios at 48 and 72 hr post-infection were higher for ZiVa at nearly all doses. ZiVa was superior to GE as it was able to detect SEAP at 48 hr, as well as when lower numbers of 293TT cells were used. The ability of ZiVa to quantitate HPV-16 and -18 neutralizing antibody titers was tested using sera from Cervarix® immunized individuals. Spearman rank correlational analyses showed excellent correlations between the titers obtained with ZiVa and GE for anti-HPV16 (r = 0.9822, p < 0.0001) and anti-HPV18 (r = 0.9832, p < 0.0001) antibodies. We concluded that ZiVa is superior to GE in detecting SEAP, and the antibody titers in sera of vaccinated individuals were similar to those obtained with GE. Thus, Ziva is a suitable alternative to GE.
Keywords: Cervarix®; ELISA, enzyme-linked immunosorbent assay; GE, Great EscAPe™ SEAP Chemiluminescence Kit 2.0; GFP, green fluorescent protein; Gardasil®; HPV, human papillomavirus; L1 and L2, late 1 and late 2 proteins; PsV, pseudovirion; RLU, relative-light-unit; SEAP; VLP, virus-like-particle; ZiVa; ZiVa, ZiVa Ultra SEAP Plus Assay; human papillomavirus; vaccine.
Figures
Similar articles
-
Immunogenicity assessment of HPV16/18 vaccine using the glutathione S-transferase L1 multiplex serology assay.Hum Vaccin Immunother. 2014;10(10):2965-74. doi: 10.4161/21645515.2014.972811. Hum Vaccin Immunother. 2014. PMID: 25483632 Free PMC article.
-
Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes.Vaccine. 2014 Sep 15;32(41):5357-62. doi: 10.1016/j.vaccine.2014.07.014. Epub 2014 Jul 18. Vaccine. 2014. PMID: 25045814
-
Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial.J Infect Dis. 2014 Apr 15;209(8):1165-73. doi: 10.1093/infdis/jit657. Epub 2013 Nov 23. J Infect Dis. 2014. PMID: 24273179 Clinical Trial.
-
Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18.Virology. 2004 Apr 10;321(2):205-16. doi: 10.1016/j.virol.2003.12.027. Virology. 2004. PMID: 15051381
-
Optimization and validation of a high throughput method for detecting neutralizing antibodies against human papillomavirus (HPV) based on pseudovirons.J Med Virol. 2014 Sep;86(9):1542-55. doi: 10.1002/jmv.23995. Epub 2014 Jun 4. J Med Virol. 2014. PMID: 24895216
Cited by
-
Production of Furin-Cleaved Papillomavirus Pseudovirions and Their Use for In Vitro Neutralization Assays of L1- or L2-Specific Antibodies.Curr Protoc Microbiol. 2015 Aug 3;38:14B.5.1-26. doi: 10.1002/9780471729259.mc14b05s38. Curr Protoc Microbiol. 2015. PMID: 26237105 Free PMC article.
References
-
- Cates W, Jr. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. american social health association panel. Sex Transm Dis 1999; 26:S2-7; PMID:10227693; http://dx.doi.org/10.1097/00007435-199904001-00002 - DOI - PubMed
-
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010; 401:70-9; PMID:20206957; http://dx.doi.org/10.1016/j.virol.2010.02.002 - DOI - PMC - PubMed
-
- Einstein MH, Schiller JT, Viscidi RP, Strickler HD, Coursaget P, Tan T, Halsey N, Jenkins D. Clinician's guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis 2009; 9:347-56; PMID:19467474; http://dx.doi.org/10.1016/S1473-3099(09)70108-2 - DOI - PubMed
-
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology 2009; 384:260-5; PMID:19135222; http://dx.doi.org/10.1016/j.virol.2008.11.046 - DOI - PubMed
-
- Bosch FX, de Sanjose S. Chapter 1: human papillomavirus and cervical cancer–burden and assessment of causality. J Natl Cancer Inst Monogr 2003:3-13; PMID:12807939; http://dx.doi.org/10.1093/oxfordjournals.jncimonographs.a003479 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
