A new method to image heme-Fe, total Fe, and aggregated protein levels after intracerebral hemorrhage
- PMID: 25695130
- PMCID: PMC4490904
- DOI: 10.1021/acschemneuro.5b00037
A new method to image heme-Fe, total Fe, and aggregated protein levels after intracerebral hemorrhage
Abstract
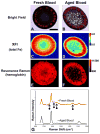
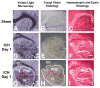
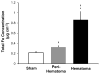
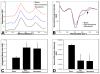
An intracerebral hemorrhage (ICH) is a devastating stroke that results in high mortality and significant disability in survivors. Unfortunately, the underlying mechanisms of this injury are not yet fully understood. After the primary (mechanical) trauma, secondary degenerative events contribute to ongoing cell death in the peri-hematoma region. Oxidative stress is thought to be a key reason for this delayed injury, which is likely due to free-Fe-catalyzed free radical reactions. Unfortunately, this is difficult to prove with conventional biochemical assays that fail to differentiate between alterations that occur within the hematoma and peri-hematoma zone. This is a critical limitation, as the hematoma contains tissue severely damaged by the initial hemorrhage and is unsalvageable, whereas the peri-hematoma region is less damaged but at risk from secondary degenerative events. Such events include oxidative stress mediated by free Fe presumed to originate from hemoglobin breakdown. Therefore, minimizing the damage caused by oxidative stress following hemoglobin breakdown and Fe release is a major therapeutic target. However, the extent to which free Fe contributes to the pathogenesis of ICH remains unknown. This investigation used a novel imaging approach that employed resonance Raman spectroscopic mapping of hemoglobin, X-ray fluorescence microscopic mapping of total Fe, and Fourier transform infrared spectroscopic imaging of aggregated protein following ICH in rats. This multimodal spectroscopic approach was used to accurately define the hematoma/peri-hematoma boundary and quantify the Fe concentration and the relative aggregated protein content, as a marker of oxidative stress, within each region. The results revealed total Fe is substantially increased in the hematoma (0.90 μg cm(-2)), and a subtle but significant increase in Fe that is not in the chemical form of hemoglobin is present within the peri-hematoma zone (0.32 μg cm(-2)) within 1 day of ICH, relative to sham animals (0.22 μg cm(-2)). Levels of aggregated protein were significantly increased within both the hematoma (integrated band area 0.10 AU) and peri-hematoma zone (integrated band area 0.10 AU) relative to sham animals (integrated band area 0.056 AU), but no significant difference in aggregated protein content was observed between the hematoma and peri-hematoma zone. This result suggests that the chemical form of Fe and its ability to generate free radicals is likely to be a more critical predictor of tissue damage than the total Fe content of the tissue. Furthermore, this article describes a novel approach to colocalize nonheme Fe and aggregated protein in the peri-hematoma zone following ICH, a significant methodological advancement for the field.
Keywords: Spectroscopic imaging; hemoglobin; intracerebral hemorrhage; iron; oxidative stress; stroke.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Rehabilitation Augments Hematoma Clearance and Attenuates Oxidative Injury and Ion Dyshomeostasis After Brain Hemorrhage.Stroke. 2017 Jan;48(1):195-203. doi: 10.1161/STROKEAHA.116.015404. Epub 2016 Nov 29. Stroke. 2017. PMID: 27899761
-
Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome.Exp Neurol. 2012 Mar;234(1):136-43. doi: 10.1016/j.expneurol.2011.12.030. Epub 2011 Dec 27. Exp Neurol. 2012. PMID: 22226595 Free PMC article.
-
After Intracerebral Hemorrhage, Oligodendrocyte Precursors Proliferate and Differentiate Inside White-Matter Tracts in the Rat Striatum.Transl Stroke Res. 2016 Jun;7(3):192-208. doi: 10.1007/s12975-015-0445-3. Epub 2016 Jan 8. Transl Stroke Res. 2016. PMID: 26743212 Free PMC article.
-
Lactoferrin and hematoma detoxification after intracerebral hemorrhage.Biochem Cell Biol. 2021 Feb;99(1):97-101. doi: 10.1139/bcb-2020-0116. Epub 2020 Sep 4. Biochem Cell Biol. 2021. PMID: 32886889 Review.
-
Oxidative Stress in Intracerebral Hemorrhage: Sources, Mechanisms, and Therapeutic Targets.Oxid Med Cell Longev. 2016;2016:3215391. doi: 10.1155/2016/3215391. Epub 2015 Dec 30. Oxid Med Cell Longev. 2016. PMID: 26843907 Free PMC article. Review.
Cited by
-
Low-density lipoprotein receptor-related protein-1 facilitates heme scavenging after intracerebral hemorrhage in mice.J Cereb Blood Flow Metab. 2017 Apr;37(4):1299-1310. doi: 10.1177/0271678X16654494. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27317656 Free PMC article.
-
Examining potential side effects of therapeutic hypothermia in experimental intracerebral hemorrhage.J Cereb Blood Flow Metab. 2017 Aug;37(8):2975-2986. doi: 10.1177/0271678X16681312. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27899766 Free PMC article.
-
Fourier-Transform Infrared Imaging Spectroscopy and Laser Ablation -ICPMS New Vistas for Biochemical Analyses of Ischemic Stroke in Rat Brain.Front Neurosci. 2018 Sep 19;12:647. doi: 10.3389/fnins.2018.00647. eCollection 2018. Front Neurosci. 2018. PMID: 30283295 Free PMC article.
-
Tracking elemental changes in an ischemic stroke model with X-ray fluorescence imaging.Sci Rep. 2020 Oct 20;10(1):17868. doi: 10.1038/s41598-020-74698-2. Sci Rep. 2020. PMID: 33082455 Free PMC article.
-
Investigation of the Effect of PD-L1 Blockade on Triple Negative Breast Cancer Cells Using Fourier Transform Infrared Spectroscopy.Vaccines (Basel). 2019 Sep 9;7(3):109. doi: 10.3390/vaccines7030109. Vaccines (Basel). 2019. PMID: 31505846 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

