Orientia tsutsugamushi ankyrin repeat-containing protein family members are Type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum
- PMID: 25692099
- PMCID: PMC4315096
- DOI: 10.3389/fcimb.2014.00186
Orientia tsutsugamushi ankyrin repeat-containing protein family members are Type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum
Abstract
Scrub typhus is an understudied, potentially fatal infection that threatens one billion persons in the Asia-Pacific region. How the causative obligate intracellular bacterium, Orientia tsutsugamushi, facilitates its intracellular survival and pathogenesis is poorly understood. Many intracellular bacterial pathogens utilize the Type 1 (T1SS) or Type 4 secretion system (T4SS) to translocate ankyrin repeat-containing proteins (Anks) that traffic to distinct subcellular locations and modulate host cell processes. The O. tsutsugamushi genome encodes one of the largest known bacterial Ank repertoires plus T1SS and T4SS components. Whether these potential virulence factors are expressed during infection, how the Anks are potentially secreted, and to where they localize in the host cell are not known. We determined that O. tsutsugamushi transcriptionally expresses 20 unique ank genes as well as genes for both T1SS and T4SS during infection of mammalian host cells. Examination of the Anks' C-termini revealed that the majority of them resemble T1SS substrates. Escherichia coli expressing a functional T1SS was able to secrete chimeric hemolysin proteins bearing the C-termini of 19 of 20 O. tsutsugamushi Anks in an HlyBD-dependent manner. Thus, O. tsutsugamushi Anks C-termini are T1SS-compatible. Conversely, Coxiella burnetii could not secrete heterologously expressed Anks in a T4SS-dependent manner. Analysis of the subcellular distribution patterns of 20 ectopically expressed Anks revealed that, while 6 remained cytosolic or trafficked to the nucleus, 14 localized to, and in some cases, altered the morphology of the endoplasmic reticulum. This study identifies O. tsutsugamushi Anks as T1SS substrates and indicates that many display a tropism for the host cell secretory pathway.
Keywords: ER-tropic effector; Rickettsia; ankyrin repeat; bacterial effector; bacterial secretion; intracellular bacteria; scrub typhus; secretory pathway.
Figures


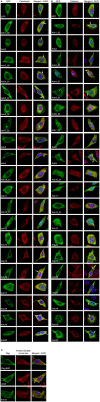
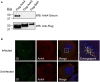
Similar articles
-
Orientia tsutsugamushi: An Unusual Intracellular Bacteria-Adaptation Strategies, Available Antibiotics, and Alternatives for Treatment.Curr Microbiol. 2024 Jun 21;81(8):236. doi: 10.1007/s00284-024-03754-1. Curr Microbiol. 2024. PMID: 38907107 Review.
-
Orientia tsutsugamushi Strain Ikeda Ankyrin Repeat-Containing Proteins Recruit SCF1 Ubiquitin Ligase Machinery via Poxvirus-Like F-Box Motifs.J Bacteriol. 2015 Oct;197(19):3097-109. doi: 10.1128/JB.00276-15. Epub 2015 Jul 13. J Bacteriol. 2015. PMID: 26170417 Free PMC article.
-
Orientia tsutsugamushi uses two Ank effectors to modulate NF-κB p65 nuclear transport and inhibit NF-κB transcriptional activation.PLoS Pathog. 2018 May 7;14(5):e1007023. doi: 10.1371/journal.ppat.1007023. eCollection 2018 May. PLoS Pathog. 2018. PMID: 29734393 Free PMC article.
-
Functional Characterization of Non-Ankyrin Repeat Domains of Orientia tsutsugamushi Ank Effectors Reveals Their Importance for Molecular Pathogenesis.Infect Immun. 2022 May 19;90(5):e0062821. doi: 10.1128/iai.00628-21. Epub 2022 Apr 18. Infect Immun. 2022. PMID: 35435726 Free PMC article.
-
Subversion of host cell signaling by Orientia tsutsugamushi.Microbes Infect. 2011 Jul;13(7):638-48. doi: 10.1016/j.micinf.2011.03.003. Epub 2011 Mar 31. Microbes Infect. 2011. PMID: 21458586 Review.
Cited by
-
Orientia tsutsugamushi: An Unusual Intracellular Bacteria-Adaptation Strategies, Available Antibiotics, and Alternatives for Treatment.Curr Microbiol. 2024 Jun 21;81(8):236. doi: 10.1007/s00284-024-03754-1. Curr Microbiol. 2024. PMID: 38907107 Review.
-
Orientia tsutsugamushi: A neglected but fascinating obligate intracellular bacterial pathogen.PLoS Pathog. 2017 Dec 7;13(12):e1006657. doi: 10.1371/journal.ppat.1006657. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29216334 Free PMC article. Review. No abstract available.
-
"Candidatus Mesenet longicola": Novel Endosymbionts of Brontispa longissima that Induce Cytoplasmic Incompatibility.Microb Ecol. 2021 Aug;82(2):512-522. doi: 10.1007/s00248-021-01686-y. Epub 2021 Jan 16. Microb Ecol. 2021. PMID: 33454808
-
crANKing up the infection: ankyrin domains in Rickettsiales and their role in host manipulation.Infect Immun. 2024 Oct 15;92(10):e0005924. doi: 10.1128/iai.00059-24. Epub 2024 Aug 30. Infect Immun. 2024. PMID: 39212405 Review.
-
Rickettsia-host interaction: strategies of intracytosolic host colonization.Pathog Dis. 2021 Apr 5;79(4):ftab015. doi: 10.1093/femspd/ftab015. Pathog Dis. 2021. PMID: 33705517 Free PMC article. Review.
References
-
- Al-Khodor S., Price C. T., Habyarimana F., Kalia A., Abu Kwaik Y. (2008). A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol. Microbiol. 70, 908–923. 10.1111/j.1365-2958.2008.06453.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

