Neural Regulation of CNS Angiogenesis During Development
- PMID: 25691893
- PMCID: PMC4327907
- DOI: 10.1007/s11515-014-1331-y
Neural Regulation of CNS Angiogenesis During Development
Abstract
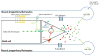
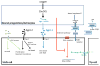
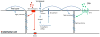
Vertebrates have evolved a powerful vascular system that involves close interactions between blood vessels and target tissues. Vascular biology had been mostly focused on the study of blood vessels for decades, which has generated large bodies of knowledge on vascular cell development, function and pathology. We argue that the prime time has arrived for vascular research on vessel-tissue interactions, especially target tissue regulation of vessel development. The central nervous system (CNS) requires a highly efficient vascular system for oxygen and nutrient transport as well as waste disposal. Therefore, neurovascular interaction is an excellent entry point to understanding target tissue regulation of blood vessel development. In this review, we summarize signaling pathways that transmit information from neural cells to blood vessels during development and the mechanisms by which they regulate each step of CNS angiogenesis. We also review important mechanisms of neural regulation of blood-brain barrier establishment and maturation, highlighting different functions of neural progenitor cells and pericytes. Finally, we evaluate potential contribution of malfunctioning neurovascular signaling to the development of brain vascular diseases and discuss how neurovascular interactions could be involved in brain tumor angiogenesis.
Figures
Similar articles
-
Neural Regulation of Vascular Development: Molecular Mechanisms and Interactions.Biomolecules. 2024 Aug 8;14(8):966. doi: 10.3390/biom14080966. Biomolecules. 2024. PMID: 39199354 Free PMC article. Review.
-
Cross-talk between blood vessels and neural progenitors in the developing brain.Neuronal Signal. 2018 Mar 30;2(1):NS20170139. doi: 10.1042/NS20170139. eCollection 2018 Mar. Neuronal Signal. 2018. PMID: 32714582 Free PMC article. Review.
-
Neurovascular development and links to disease.Cell Mol Life Sci. 2013 May;70(10):1675-84. doi: 10.1007/s00018-013-1277-5. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23475065 Free PMC article. Review.
-
Neurovascular development: The beginning of a beautiful friendship.Cell Adh Migr. 2009 Apr-Jun;3(2):199-204. doi: 10.4161/cam.3.2.8397. Epub 2009 Apr 13. Cell Adh Migr. 2009. PMID: 19363295 Free PMC article. Review.
-
Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia.Front Aging Neurosci. 2020 Apr 14;12:80. doi: 10.3389/fnagi.2020.00080. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32317958 Free PMC article. Review.
Cited by
-
Ontogeny of adult neural stem cells in the mammalian brain.Curr Top Dev Biol. 2021;142:67-98. doi: 10.1016/bs.ctdb.2020.11.002. Epub 2020 Dec 17. Curr Top Dev Biol. 2021. PMID: 33706926 Free PMC article. Review.
-
Harnessing region-specific neurovascular signaling to promote germinal matrix vessel maturation and hemorrhage prevention.Dis Model Mech. 2019 Nov 11;12(11):dmm041228. doi: 10.1242/dmm.041228. Dis Model Mech. 2019. PMID: 31601549 Free PMC article.
-
Exploring the origin of the cancer stem cell niche and its role in anti-angiogenic treatment for glioblastoma.Front Oncol. 2022 Aug 25;12:947634. doi: 10.3389/fonc.2022.947634. eCollection 2022. Front Oncol. 2022. PMID: 36091174 Free PMC article. Review.
-
Vascularizing the brain in vitro.iScience. 2022 Mar 17;25(4):104110. doi: 10.1016/j.isci.2022.104110. eCollection 2022 Apr 15. iScience. 2022. PMID: 35378862 Free PMC article. Review.
-
αvβ8 integrin adhesion and signaling pathways in development, physiology and disease.J Cell Sci. 2020 Jun 15;133(12):jcs239434. doi: 10.1242/jcs.239434. J Cell Sci. 2020. PMID: 32540905 Free PMC article. Review.
References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Rev. 2006;7:41–53. - PubMed
-
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. - PubMed
-
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. - PubMed
-
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, MacDonald LE, Thurston G, Daly C, Lin HC, Economides AN, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gale NW. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein–coupled receptor. Proc Natl Acad Sci USA. 2011;108:2807–2812. - PMC - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
