Antigenicity and immunogenicity of a trimeric envelope protein from an Indian clade C HIV-1 isolate
- PMID: 25691567
- PMCID: PMC4423705
- DOI: 10.1074/jbc.M114.621185
Antigenicity and immunogenicity of a trimeric envelope protein from an Indian clade C HIV-1 isolate
Abstract
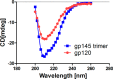
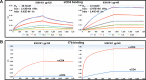
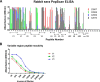
Human immunodeficiency virus type 1 (HIV-1) isolates from India mainly belong to clade C and are quite distinct from clade C isolates from Africa in terms of their phylogenetic makeup, serotype, and sensitivity to known human broadly neutralizing monoclonal antibodies. Because many of these properties are associated with the envelope proteins of HIV-1, it is of interest to study the envelope proteins of Indian clade C isolates as part of the ongoing efforts to develop a vaccine against HIV-1. To this end, we purified trimeric uncleaved gp145 of a CCR5 tropic Indian clade C HIV-1 (93IN101) from the conditioned medium of 293 cells. The purified protein was shown to be properly folded with stable structure by circular dichroism. Conformational integrity was further demonstrated by its high affinity binding to soluble CD4, CD4 binding site antibodies such as b12 and VRC01, quaternary epitope-specific antibody PG9, and CD4-induced epitope-specific antibody 17b. Sera from rabbits immunized with gp145 elicited high titer antibodies to various domains of gp120 and neutralized a broad spectrum of clade B and clade C HIV-1 isolates. Similar to other clade B and clade C envelope immunogens, most of the Tier 1 neutralizing activity could be absorbed with the V3-specific peptide. Subsequent boosting of these rabbits with a clade B HIV-1 Bal gp145 resulted in an expanded breadth of neutralization of HIV-1 isolates. The present study strongly supports the inclusion of envelopes from Indian isolates in a future mixture of HIV-1 vaccines.
Keywords: AIDS; Antigen; Cell Surface Protein; Human Immunodeficiency Virus (HIV); Vaccine Development.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Membrane bound Indian clade C HIV-1 envelope antigen induces antibodies to diverse and conserved epitopes upon DNA prime/protein boost in rabbits.Vaccine. 2016 May 5;34(21):2444-2452. doi: 10.1016/j.vaccine.2016.03.062. Epub 2016 Mar 28. Vaccine. 2016. PMID: 27032514
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
Rapid Induction of Multifunctional Antibodies in Rabbits and Macaques by Clade C HIV-1 CAP257 Envelopes Circulating During Epitope-Specific Neutralization Breadth Development.Front Immunol. 2020 Jun 2;11:984. doi: 10.3389/fimmu.2020.00984. eCollection 2020. Front Immunol. 2020. PMID: 32582155 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Hyperglycosylated stable core immunogens designed to present the CD4 binding site are preferentially recognized by broadly neutralizing antibodies.J Virol. 2014 Dec;88(24):14002-16. doi: 10.1128/JVI.02614-14. Epub 2014 Sep 24. J Virol. 2014. PMID: 25253346 Free PMC article.
Cited by
-
Broad and potent cross clade neutralizing antibodies with multiple specificities in the plasma of HIV-1 subtype C infected individuals.Sci Rep. 2017 Apr 24;7:46557. doi: 10.1038/srep46557. Sci Rep. 2017. PMID: 28436427 Free PMC article. Clinical Trial.
-
Enhanced Immune Responses to HIV-1 Envelope Elicited by a Vaccine Regimen Consisting of Priming with Newcastle Disease Virus Expressing HIV gp160 and Boosting with gp120 and SOSIP gp140 Proteins.J Virol. 2015 Nov 18;90(3):1682-6. doi: 10.1128/JVI.02847-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26581986 Free PMC article.
-
DNA prime/protein boost vaccination elicits robust humoral response in rhesus macaques using oligomeric simian immunodeficiency virus envelope and Advax delta inulin adjuvant.J Gen Virol. 2017 Aug;98(8):2143-2155. doi: 10.1099/jgv.0.000863. Epub 2017 Jul 31. J Gen Virol. 2017. PMID: 28758637 Free PMC article.
-
Structure-based Design of Cyclically Permuted HIV-1 gp120 Trimers That Elicit Neutralizing Antibodies.J Biol Chem. 2017 Jan 6;292(1):278-291. doi: 10.1074/jbc.M116.725614. Epub 2016 Nov 22. J Biol Chem. 2017. PMID: 27879316 Free PMC article.
-
Optimizing the Production of gp145, an HIV-1 Envelope Glycoprotein Vaccine Candidate and Its Encapsulation in Guanosine Microparticles.Vaccines (Basel). 2023 May 12;11(5):975. doi: 10.3390/vaccines11050975. Vaccines (Basel). 2023. PMID: 37243079 Free PMC article.
References
-
- Rosenberg E. S., Billingsley J. M., Caliendo A. M., Boswell S. L., Sax P. E., Kalams S. A., Walker B. D. (1997) Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278, 1447–1450 - PubMed
-
- Matano T., Kobayashi M., Igarashi H., Takeda A., Nakamura H., Kano M., Sugimoto C., Mori K., Iida A., Hirata T., Hasegawa M., Yuasa T., Miyazawa M., Takahashi Y., Yasunami M., Kimura A., O'Connor D. H., Watkins D. I., Nagai Y. (2004) Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199, 1709–1718 - PMC - PubMed
-
- Amara R. R., Sharma S., Patel M., Smith J. M., Chennareddi L., Herndon J. G., Robinson H. L. (2005) Studies on the cross-clade and cross-species conservation of HIV-1 Gag-specific CD8 and CD4 T cell responses elicited by a clade B DNA/MVA vaccine in macaques. Virology 334, 124–133 - PubMed
-
- Pitisuttithum P., Gilbert P., Gurwith M., Heyward W., Martin M., van Griensven F., Hu D., Tappero J. W., Choopanya K. (2006) Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194, 1661–1671 - PubMed
-
- Gilbert P. B., Peterson M. L., Follmann D., Hudgens M. G., Francis D. P., Gurwith M., Heyward W. L., Jobes D. V., Popovic V., Self S. G., Sinangil F., Burke D., Berman P. W. (2005) Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 191, 666–677 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

