The multiple cellular functions of the oncoprotein Golgi phosphoprotein 3
- PMID: 25691054
- PMCID: PMC4414131
- DOI: 10.18632/oncotarget.3051
The multiple cellular functions of the oncoprotein Golgi phosphoprotein 3
Abstract
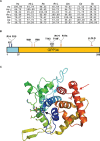
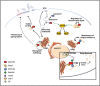
The highly conserved Golgi phosphoprotein 3 (GOLPH3) protein, a component of Trans-Golgi Network (TGN), has been defined as a "first-in-class Golgi oncoprotein" and characterized as a Phosphatidylinositol 4-phosphate [PI(4)P] effector at the Golgi. GOLPH3 is commonly amplified in several solid tumors. Furthermore this protein has been associated with poor prognosis in many cancers. Highly conserved from yeast to humans, GOLPH3 provides an essential function in vesicle trafficking and Golgi structure. Recent data have also implicated this oncoprotein in regulation of cytokinesis, modulation of mitochondrial mass and cellular response to DNA damage. A minute dissection of the molecular pathways that require GOLPH3 protein will be helpful to develop new therapeutic cancer strategies.
Figures

Similar articles
-
Cigarette Smoking Condensate Disrupts Endoplasmic Reticulum-Golgi Network Homeostasis Through GOLPH3 Expression in Normal Lung Epithelial Cells.Nicotine Tob Res. 2016 Sep;18(9):1877-1885. doi: 10.1093/ntr/ntw079. Epub 2016 Mar 31. Nicotine Tob Res. 2016. PMID: 27611309
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Phospholipase C beta3 is a key component in the Gbetagamma/PKCeta/PKD-mediated regulation of trans-Golgi network to plasma membrane transport.Biochem J. 2007 Aug 15;406(1):157-65. doi: 10.1042/BJ20070359. Biochem J. 2007. PMID: 17492941 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Linking GOLPH3 and Extracellular Vesicles Content-a Potential New Route in Cancer Physiopathology and a Promising Therapeutic Target is in Sight?Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221135724. doi: 10.1177/15330338221135724. Technol Cancer Res Treat. 2022. PMID: 36320176 Free PMC article. Review.
-
The knocking down of the oncoprotein Golgi phosphoprotein 3 in T98G cells of glioblastoma multiforme disrupts cell migration by affecting focal adhesion dynamics in a focal adhesion kinase-dependent manner.PLoS One. 2019 Feb 19;14(2):e0212321. doi: 10.1371/journal.pone.0212321. eCollection 2019. PLoS One. 2019. PMID: 30779783 Free PMC article.
-
The function of apolipoproteins L (APOLs): relevance for kidney disease, neurotransmission disorders, cancer and viral infection.FEBS J. 2021 Jan;288(2):360-381. doi: 10.1111/febs.15444. Epub 2020 Jun 25. FEBS J. 2021. PMID: 32530132 Free PMC article. Review.
-
GOLPH3 promotes glioma progression via facilitating JAK2-STAT3 pathway activation.J Neurooncol. 2018 Sep;139(2):269-279. doi: 10.1007/s11060-018-2884-7. Epub 2018 Apr 30. J Neurooncol. 2018. PMID: 29713848
-
Golgi phosphoprotein 3 promotes glioma progression via inhibiting Rab5-mediated endocytosis and degradation of epidermal growth factor receptor.Neuro Oncol. 2017 Nov 29;19(12):1628-1639. doi: 10.1093/neuonc/nox104. Neuro Oncol. 2017. PMID: 28575494 Free PMC article.
References
-
- Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

