Preventing effect of L-type calcium channel blockade on electrophysiological alterations in dentate gyrus granule cells induced by entorhinal amyloid pathology
- PMID: 25689857
- PMCID: PMC4331091
- DOI: 10.1371/journal.pone.0117555
Preventing effect of L-type calcium channel blockade on electrophysiological alterations in dentate gyrus granule cells induced by entorhinal amyloid pathology
Abstract
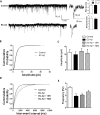
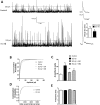
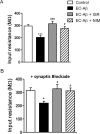
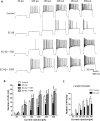
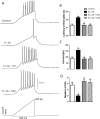
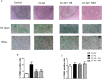
The entorhinal cortex (EC) is one of the earliest affected brain regions in Alzheimer's disease (AD). EC-amyloid pathology induces synaptic failure in the dentate gyrus (DG) with resultant behavioral impairment, but there is little known about its impact on neuronal properties in the DG. It is believed that calcium dyshomeostasis plays a pivotal role in the etiology of AD. Here, the effect of the EC amyloid pathogenesis on cellular properties of DG granule cells and also possible neuroprotective role of L-type calcium channel blockers (CCBs), nimodipine and isradipine, were investigated. The amyloid beta (Aβ) 1-42 was injected bilaterally into the EC of male rats and one week later, electrophysiological properties of DG granule cells were assessed. Voltage clamp recording revealed appearance of giant sIPSC in combination with a decrease in sEPSC frequency which was partially reversed by CCBs in granule cells from Aβ treated rats. EC amyloid pathogenesis induced a significant reduction of input resistance (Rin) accompanied by a profound decreased excitability in the DG granule cells. However, daily administration of CCBs, isradipine or nimodipine (i.c.v. for 6 days), almost preserved the normal excitability against Aβ. In conclusion, lower tendency to fire AP along with reduced Rin suggest that DG granule cells might undergo an alteration in the membrane ion channel activities which finally lead to the behavioral deficits observed in animal models and patients with early-stage Alzheimer's disease.
Conflict of interest statement
Figures

Similar articles
-
Neuroprotective Effects of Ferrostatin and Necrostatin Against Entorhinal Amyloidopathy-Induced Electrophysiological Alterations Mediated by voltage-gated Ca2+ Channels in the Dentate Gyrus Granular Cells.Neurochem Res. 2024 Jan;49(1):99-116. doi: 10.1007/s11064-023-04006-7. Epub 2023 Aug 24. Neurochem Res. 2024. PMID: 37615884
-
Calcium channel blockade attenuates abnormal synaptic transmission in the dentate gyrus elicited by entorhinal amyloidopathy.Synapse. 2016 Oct;70(10):408-17. doi: 10.1002/syn.21915. Epub 2016 Jul 7. Synapse. 2016. PMID: 27240164
-
Decrease of high voltage Ca2+ currents in the dentate gyrus granule cells by entorhinal amyloidopathy is reversed by calcium channel blockade.Eur J Pharmacol. 2017 Jan 5;794:154-161. doi: 10.1016/j.ejphar.2016.11.032. Epub 2016 Nov 24. Eur J Pharmacol. 2017. PMID: 27889432
-
L-type calcium channel blockade alleviates molecular and reversal spatial learning and memory alterations induced by entorhinal amyloid pathology in rats.Behav Brain Res. 2013 Jan 15;237:190-9. doi: 10.1016/j.bbr.2012.09.045. Epub 2012 Sep 29. Behav Brain Res. 2013. PMID: 23032184
-
Is Vulnerability of the Dentate Gyrus to Aging and Amyloid-β1-42 Neurotoxicity Linked with Modified Extracellular Zn2+ Dynamics?Biol Pharm Bull. 2018;41(7):995-1000. doi: 10.1248/bpb.b17-00871. Biol Pharm Bull. 2018. PMID: 29962410 Review.
Cited by
-
Calcium Channel Blockade Ameliorates Endoplasmic Reticulum Stress in the Hippocampus Induced by Amyloidopathy in the Entorhinal Cortex.Iran J Pharm Res. 2019 Summer;18(3):1466-1476. doi: 10.22037/ijpr.2019.111532.13216. Iran J Pharm Res. 2019. PMID: 32641955 Free PMC article.
-
Therapeutic Strategies to Target Calcium Dysregulation in Alzheimer's Disease.Cells. 2020 Nov 20;9(11):2513. doi: 10.3390/cells9112513. Cells. 2020. PMID: 33233678 Free PMC article. Review.
-
Neuroprotective Effects of Ferrostatin and Necrostatin Against Entorhinal Amyloidopathy-Induced Electrophysiological Alterations Mediated by voltage-gated Ca2+ Channels in the Dentate Gyrus Granular Cells.Neurochem Res. 2024 Jan;49(1):99-116. doi: 10.1007/s11064-023-04006-7. Epub 2023 Aug 24. Neurochem Res. 2024. PMID: 37615884
-
T2N as a new tool for robust electrophysiological modeling demonstrated for mature and adult-born dentate granule cells.Elife. 2017 Nov 22;6:e26517. doi: 10.7554/eLife.26517. Elife. 2017. PMID: 29165247 Free PMC article.
-
Targeting microglia L-type voltage-dependent calcium channels for the treatment of central nervous system disorders.J Neurosci Res. 2021 Jan;99(1):141-162. doi: 10.1002/jnr.24585. Epub 2020 Jan 29. J Neurosci Res. 2021. PMID: 31997405 Free PMC article. Review.
References
-
- Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer's disease. Lancet 368: 387–403. - PubMed
-
- Hardy J (2006) Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis 9: 151–153. - PubMed
-
- Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 120: 545–555. - PubMed
-
- Walsh DM, Selkoe DJ (2004) Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron 44: 181–193. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

