Shed syndecan-2 enhances tumorigenic activities of colon cancer cells
- PMID: 25686828
- PMCID: PMC4414160
- DOI: 10.18632/oncotarget.2885
Shed syndecan-2 enhances tumorigenic activities of colon cancer cells
Abstract
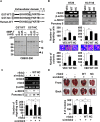
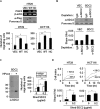
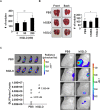
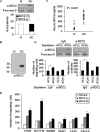
Because earlier studies showed the cell surface heparan sulfate proteoglycan, syndecan-2, sheds from colon cancer cells in culture, the functional roles of shed syndecan-2 were assessed. A non-cleavable mutant of syndecan-2 in which the Asn148-Leu149 residues were replaced with Asn148-Ile149, had decreased shedding, less cancer-associated activities of syndecan-2 in vitro, and less syndecan-2-mediated metastasis of mouse melanoma cells in vivo, suggesting the importance of shedding on syndecan-2-mediated pro-tumorigenic functions. Indeed, shed syndecan-2 from cancer-conditioned media and recombinant shed syndecan-2 enhanced cancer-associated activities, and depletion of shed syndecan-2 abolished these effects. Similarly, shed syndecan-2 was detected from sera of patients from advanced carcinoma (625.9 ng/ml) and promoted cancer-associated activities. Furthermore, a series of syndecan-2 deletion mutants showed that the tumorigenic activity of shed syndecan-2 resided in the C-terminus of the extracellular domain and a shed syndecan-2 synthetic peptide (16 residues) was sufficient to establish subcutaneous primary growth of HT29 colon cancer cells, pulmonary metastases (B16F10 cells), and primary intrasplenic tumor growth and liver metastases (4T1 cells). Taken together, these results demonstrate that shed syndecan-2 directly enhances colon cancer progression and may be a promising therapeutic target for controlling colon cancer development.
Figures

Similar articles
-
The extracellular domain of syndecan-2 regulates the interaction of HCT116 human colon carcinoma cells with fibronectin.Biochem Biophys Res Commun. 2013 Feb 15;431(3):415-20. doi: 10.1016/j.bbrc.2012.12.155. Epub 2013 Jan 16. Biochem Biophys Res Commun. 2013. PMID: 23333331
-
Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1.Biochem Biophys Res Commun. 2011 May 27;409(1):148-53. doi: 10.1016/j.bbrc.2011.04.135. Epub 2011 May 5. Biochem Biophys Res Commun. 2011. PMID: 21569759
-
The matrix metalloproteinase-7 regulates the extracellular shedding of syndecan-2 from colon cancer cells.Biochem Biophys Res Commun. 2012 Jan 27;417(4):1260-4. doi: 10.1016/j.bbrc.2011.12.120. Epub 2011 Dec 29. Biochem Biophys Res Commun. 2012. PMID: 22227189
-
Emerging roles of syndecan 2 in epithelial and mesenchymal cancer progression.IUBMB Life. 2017 Nov;69(11):824-833. doi: 10.1002/iub.1678. Epub 2017 Sep 23. IUBMB Life. 2017. PMID: 28940845 Review.
-
New insights into syndecan-2 expression and tumourigenic activity in colon carcinoma cells.J Mol Histol. 2004 Mar;35(3):319-26. doi: 10.1023/b:hijo.0000032363.78829.4e. J Mol Histol. 2004. PMID: 15339051 Review.
Cited by
-
Heparan Sulfate Proteoglycans in Human Colorectal Cancer.Anal Cell Pathol (Amst). 2018 Jun 20;2018:8389595. doi: 10.1155/2018/8389595. eCollection 2018. Anal Cell Pathol (Amst). 2018. PMID: 30027065 Free PMC article. Review.
-
Prognostic Bone Metastasis-Associated Immune-Related Genes Regulated by Transcription Factors in Mesothelioma.Biomed Res Int. 2022 Jan 27;2022:9940566. doi: 10.1155/2022/9940566. eCollection 2022. Biomed Res Int. 2022. PMID: 35127947 Free PMC article.
-
Extended disorder at the cell surface: The conformational landscape of the ectodomains of syndecans.Matrix Biol Plus. 2021 Jul 19;12:100081. doi: 10.1016/j.mbplus.2021.100081. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34505054 Free PMC article.
-
Syndecan-2 cytoplasmic domain up-regulates matrix metalloproteinase-7 expression via the protein kinase Cγ-mediated FAK/ERK signaling pathway in colon cancer.J Biol Chem. 2017 Sep 29;292(39):16321-16332. doi: 10.1074/jbc.M117.793752. Epub 2017 Aug 16. J Biol Chem. 2017. PMID: 28821612 Free PMC article.
-
Heparan sulfate proteoglycans as targets for cancer therapy: a review.Cancer Biol Ther. 2020 Dec 1;21(12):1087-1094. doi: 10.1080/15384047.2020.1838034. Epub 2020 Nov 12. Cancer Biol Ther. 2020. PMID: 33180600 Free PMC article. Review.
References
-
- Fakih MM. KRAS mutation screening in colorectal cancer: From paper to practice. Clin Colorectal Cancer. 2010;9:22–30. - PubMed
-
- Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev. 2010;29:49–60. - PubMed
-
- Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348–1360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

