Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation
- PMID: 25680967
- PMCID: PMC4657752
- DOI: 10.1016/j.clim.2015.02.001
Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation
Abstract
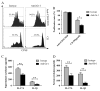
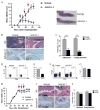
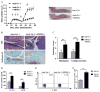
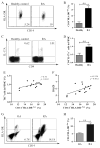
Myeloid-derived suppressor cells (MDSC) and Th17 cells were found to expand in collagen-induced arthritis (CIA) significantly. Two subsets of MDSC, polymorphonuclear (PMN) and mononuclear (MO), were detected and their ratios varied during the development of CIA. The depletion of MDSC in vivo resulted in suppression of T-cell proliferation and decreased IL-17A and IL-1β production. The adoptive transfer of MDSC restored the severity of arthritis and Th17 cell differentiation. The depletion of MDSCs on day 35 resulted in arthritis amelioration without reaching a significant difference. Furthermore, MDSCs from CIA mice had higher production of IL-1β and promoted Th17 cell differentiation. The expansion of MDSCs in the peripheral blood of rheumatoid arthritis (RA) patients was in correlation with increased Th17 cells and disease activity DAS28. These results support the hypothesis that MDSC may play a significant proinflammatory role in the pathogenesis of CIA and RA by inducing Th17 development in an IL-1β-dependent manner.
Keywords: Collagen-induced arthritis; IL-1β; Myeloid-derived suppressor cells; Th17 cells.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
All the authors have no interests to declare.
Figures


Similar articles
-
Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis.J Immunol. 2013 Aug 1;191(3):1073-81. doi: 10.4049/jimmunol.1203535. Epub 2013 Jun 26. J Immunol. 2013. PMID: 23804709
-
IL-37 Alleviates Rheumatoid Arthritis by Suppressing IL-17 and IL-17-Triggering Cytokine Production and Limiting Th17 Cell Proliferation.J Immunol. 2015 Jun 1;194(11):5110-9. doi: 10.4049/jimmunol.1401810. Epub 2015 Apr 27. J Immunol. 2015. PMID: 25917106
-
Functional characterization of myeloid-derived suppressor cell subpopulations during the development of experimental arthritis.Eur J Immunol. 2015 Feb;45(2):464-73. doi: 10.1002/eji.201444799. Epub 2014 Nov 28. Eur J Immunol. 2015. PMID: 25352399
-
Roles of Myeloid-Derived Suppressor Cell Subpopulations in Autoimmune Arthritis.Front Immunol. 2018 Dec 4;9:2849. doi: 10.3389/fimmu.2018.02849. eCollection 2018. Front Immunol. 2018. PMID: 30564242 Free PMC article. Review.
-
The potential therapeutic role of myeloid-derived suppressor cells in autoimmune arthritis.Semin Arthritis Rheum. 2016 Feb;45(4):490-5. doi: 10.1016/j.semarthrit.2015.07.003. Epub 2015 Jul 13. Semin Arthritis Rheum. 2016. PMID: 26272193 Review.
Cited by
-
A Peripheral Blood Signature of Increased Th1 and Myeloid Cells Combined with Serum Inflammatory Mediators Is Associated with Response to Abatacept in Rheumatoid Arthritis Patients.Cells. 2023 Dec 9;12(24):2808. doi: 10.3390/cells12242808. Cells. 2023. PMID: 38132128 Free PMC article.
-
NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis.Clin Exp Immunol. 2018 Nov;194(2):231-243. doi: 10.1111/cei.13167. Epub 2018 Sep 16. Clin Exp Immunol. 2018. PMID: 30277570 Free PMC article.
-
MicroRNA Post-transcriptional Regulation of the NLRP3 Inflammasome in Immunopathologies.Front Pharmacol. 2019 May 1;10:451. doi: 10.3389/fphar.2019.00451. eCollection 2019. Front Pharmacol. 2019. PMID: 31118894 Free PMC article. Review.
-
Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases.Int J Mol Sci. 2019 Jun 13;20(12):2876. doi: 10.3390/ijms20122876. Int J Mol Sci. 2019. PMID: 31200447 Free PMC article. Review.
-
Increased Frequency of Myeloid-Derived Suppressor Cells in Myasthenia Gravis After Immunotherapy.Front Neurol. 2022 Jun 29;13:902384. doi: 10.3389/fneur.2022.902384. eCollection 2022. Front Neurol. 2022. PMID: 35847216 Free PMC article.
References
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. - PubMed
-
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
-
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28(4):445–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

