Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem
- PMID: 25679778
- PMCID: PMC4332492
- DOI: 10.1371/journal.pone.0117266
Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem
Abstract
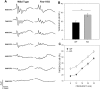
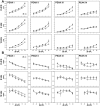
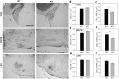
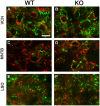
Fragile X Syndrome (FXS), a neurodevelopmental disorder, is the most prevalent single-gene cause of autism spectrum disorder. Autism has been associated with impaired auditory processing, abnormalities in the auditory brainstem response (ABR), and reduced cell number and size in the auditory brainstem nuclei. FXS is characterized by elevated cortical responses to sound stimuli, with some evidence for aberrant ABRs. Here, we assessed ABRs and auditory brainstem anatomy in Fmr1-/- mice, an animal model of FXS. We found that Fmr1-/- mice showed elevated response thresholds to both click and tone stimuli. Amplitudes of ABR responses were reduced in Fmr1-/- mice for early peaks of the ABR. The growth of the peak I response with sound intensity was less steep in mutants that in wild type mice. In contrast, amplitudes and response growth in peaks IV and V did not differ between these groups. We did not observe differences in peak latencies or in interpeak latencies. Cell size was reduced in Fmr1-/- mice in the ventral cochlear nucleus (VCN) and in the medial nucleus of the trapezoid body (MNTB). We quantified levels of inhibitory and excitatory synaptic inputs in these nuclei using markers for presynaptic proteins. We measured VGAT and VGLUT immunolabeling in VCN, MNTB, and the lateral superior olive (LSO). VGAT expression in MNTB was significantly greater in the Fmr1-/- mouse than in wild type mice. Together, these observations demonstrate that FXS affects peripheral and central aspects of hearing and alters the balance of excitation and inhibition in the auditory brainstem.
Conflict of interest statement
Figures
Similar articles
-
Developmental Emergence of Phenotypes in the Auditory Brainstem Nuclei of Fmr1 Knockout Mice.eNeuro. 2017 Dec 27;4(6):ENEURO.0264-17.2017. doi: 10.1523/ENEURO.0264-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29291238 Free PMC article.
-
Enhanced Excitatory Connectivity and Disturbed Sound Processing in the Auditory Brainstem of Fragile X Mice.J Neurosci. 2017 Aug 2;37(31):7403-7419. doi: 10.1523/JNEUROSCI.2310-16.2017. Epub 2017 Jul 3. J Neurosci. 2017. PMID: 28674175 Free PMC article.
-
Modulators of Kv3 Potassium Channels Rescue the Auditory Function of Fragile X Mice.J Neurosci. 2019 Jun 12;39(24):4797-4813. doi: 10.1523/JNEUROSCI.0839-18.2019. Epub 2019 Apr 1. J Neurosci. 2019. PMID: 30936239 Free PMC article.
-
Sensory Processing Phenotypes in Fragile X Syndrome.ASN Neuro. 2018 Jan-Dec;10:1759091418801092. doi: 10.1177/1759091418801092. ASN Neuro. 2018. PMID: 30231625 Free PMC article. Review.
-
BDNF in fragile X syndrome.Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review.
Cited by
-
Auditory Dysfunction in Animal Models of Autism Spectrum Disorder.Front Mol Neurosci. 2022 Apr 13;15:845155. doi: 10.3389/fnmol.2022.845155. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35493332 Free PMC article. Review.
-
Developmental Emergence of Phenotypes in the Auditory Brainstem Nuclei of Fmr1 Knockout Mice.eNeuro. 2017 Dec 27;4(6):ENEURO.0264-17.2017. doi: 10.1523/ENEURO.0264-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29291238 Free PMC article.
-
Neurotransmitter- and Release-Mode-Specific Modulation of Inhibitory Transmission by Group I Metabotropic Glutamate Receptors in Central Auditory Neurons of the Mouse.J Neurosci. 2018 Sep 19;38(38):8187-8199. doi: 10.1523/JNEUROSCI.0603-18.2018. Epub 2018 Aug 9. J Neurosci. 2018. PMID: 30093538 Free PMC article.
-
Abnormal development of auditory responses in the inferior colliculus of a mouse model of Fragile X Syndrome.J Neurophysiol. 2020 Jun 1;123(6):2101-2121. doi: 10.1152/jn.00706.2019. Epub 2020 Apr 22. J Neurophysiol. 2020. PMID: 32319849 Free PMC article.
-
Subtle differences in synaptic transmission in medial nucleus of trapezoid body neurons between wild-type and Fmr1 knockout mice.Brain Res. 2019 Aug 15;1717:95-103. doi: 10.1016/j.brainres.2019.04.006. Epub 2019 Apr 17. Brain Res. 2019. PMID: 31004576 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

