Heme Oxygenase-1 Regulates Myeloid Cell Trafficking in AKI
- PMID: 25677389
- PMCID: PMC4552119
- DOI: 10.1681/ASN.2014080770
Heme Oxygenase-1 Regulates Myeloid Cell Trafficking in AKI
Abstract
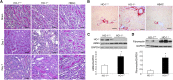
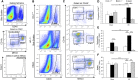
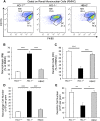
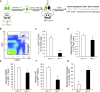
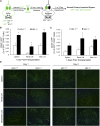
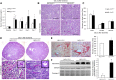
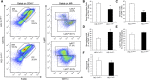
Renal ischemia-reperfusion injury is mediated by a complex cascade of events, including the immune response, that occur secondary to injury to renal epithelial cells. We tested the hypothesis that heme oxygenase-1 (HO-1) expression, which is protective in ischemia-reperfusion injury, regulates trafficking of myeloid-derived immune cells in the kidney. Age-matched male wild-type (HO-1(+/+)), HO-1-knockout (HO-1(-/-)), and humanized HO-1-overexpressing (HBAC) mice underwent bilateral renal ischemia for 10 minutes. Ischemia-reperfusion injury resulted in significantly worse renal structure and function and increased mortality in HO-1(-/-) mice. In addition, there were more macrophages (CD45(+) CD11b(hi)F4/80(lo)) and neutrophils (CD45(+) CD11b(hi) MHCII(-) Gr-1(hi)) in HO-1(-/-) kidneys than in sham and HO-1(+/+) control kidneys subjected to ischemia-reperfusion. However, ischemic injury resulted in a significant decrease in the intrarenal resident dendritic cell (DC; CD45(+)MHCII(+)CD11b(lo)F4/80(hi)) population in HO-1(-/-) kidneys compared with controls. Syngeneic transplant experiments utilizing green fluorescent protein-positive HO-1(+/+) or HO-1(-/-) donor kidneys and green fluorescent protein-negative HO-1(+/+) recipients confirmed increased migration of the resident DC population from HO-1(-/-) donor kidneys, compared to HO-1(+/+) donor kidneys, to the peripheral lymphoid organs. This effect on renal DC migration was corroborated in myeloid-specific HO-1(-/-) mice subjected to bilateral ischemia. These mice also displayed impaired renal recovery and increased fibrosis at day 7 after injury. These results highlight an important role for HO-1 in orchestrating the trafficking of myeloid cells in AKI, which may represent a key pathway for therapeutic intervention.
Keywords: ARF; heme oxygenase; macrophages.
Copyright © 2015 by the American Society of Nephrology.
Figures


Comment in
-
Myeloid Cell HO-ming in AKI.J Am Soc Nephrol. 2015 Sep;26(9):2067-9. doi: 10.1681/ASN.2015010072. Epub 2015 Feb 12. J Am Soc Nephrol. 2015. PMID: 25677391 Free PMC article. No abstract available.
Similar articles
-
Specific expression of heme oxygenase-1 by myeloid cells modulates renal ischemia-reperfusion injury.Sci Rep. 2017 Mar 15;7(1):197. doi: 10.1038/s41598-017-00220-w. Sci Rep. 2017. PMID: 28298633 Free PMC article.
-
[Heme oxygenase-1 (HO-1) gene knockout affects the balance of lung immune cell composition and aggravates inflammatory injury in lung tissues of LPS-induced acute lung injury (ALI) mice].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024 Apr;40(4):296-302. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024. PMID: 38710513 Chinese.
-
The role of heme oxygenase 1 in rapamycin-induced renal dysfunction after ischemia and reperfusion injury.Kidney Int. 2006 Nov;70(10):1742-9. doi: 10.1038/sj.ki.5001893. Epub 2006 Sep 27. Kidney Int. 2006. PMID: 17003813
-
Cytoprotective effects of heme oxygenase in acute renal failure.Contrib Nephrol. 2005;148:70-85. doi: 10.1159/000086044. Contrib Nephrol. 2005. PMID: 15912028 Review.
-
Deciphering the Role of Heme Oxygenase-1 (HO-1) Expressing Macrophages in Renal Ischemia-Reperfusion Injury.Biomedicines. 2021 Mar 16;9(3):306. doi: 10.3390/biomedicines9030306. Biomedicines. 2021. PMID: 33809696 Free PMC article. Review.
Cited by
-
Immunomodulatory Effects of Heme Oxygenase-1 in Kidney Disease.Front Med (Lausanne). 2021 Aug 24;8:708453. doi: 10.3389/fmed.2021.708453. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34504854 Free PMC article. Review.
-
Myeloid Cell HO-ming in AKI.J Am Soc Nephrol. 2015 Sep;26(9):2067-9. doi: 10.1681/ASN.2015010072. Epub 2015 Feb 12. J Am Soc Nephrol. 2015. PMID: 25677391 Free PMC article. No abstract available.
-
Divergent effects of AKI to CKD models on inflammation and fibrosis.Am J Physiol Renal Physiol. 2018 Oct 1;315(4):F1107-F1118. doi: 10.1152/ajprenal.00179.2018. Epub 2018 Jun 13. Am J Physiol Renal Physiol. 2018. PMID: 29897282 Free PMC article.
-
New insights into the role of heme oxygenase-1 in acute kidney injury.Kidney Res Clin Pract. 2020 Dec 31;39(4):387-401. doi: 10.23876/j.krcp.20.091. Kidney Res Clin Pract. 2020. PMID: 33184238 Free PMC article. Review.
-
Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury.Am J Physiol Renal Physiol. 2016 Mar 1;310(5):F385-94. doi: 10.1152/ajprenal.00335.2015. Epub 2015 Dec 16. Am J Physiol Renal Physiol. 2016. PMID: 26672618 Free PMC article.
References
-
- Guo JK, Cantley LG: Cellular maintenance and repair of the kidney. Annu Rev Physiol 72: 357–376, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

