MDA5-filament, dynamics and disease
- PMID: 25676875
- PMCID: PMC4470736
- DOI: 10.1016/j.coviro.2015.01.011
MDA5-filament, dynamics and disease
Abstract
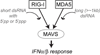
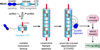
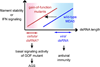
Melanoma Differentiation-Associated gene 5 (MDA5), encoded by the gene IFIH1, is a cytoplasmic sensor for viral double-stranded RNAs (dsRNAs). MDA5 activates the type I interferon signaling pathway upon detection of long viral dsRNA generated during replication of picornaviruses. Studies have shown that MDA5 forms a filament along the length of dsRNA and utilizes ATP-dependent filament dynamics to discriminate between self versus non-self on the basis of dsRNA length. This review summarizes our current understanding of how the MDA5 filament assembles and disassembles, how this filament dynamics are utilized in dsRNA length-dependent signaling, and how dysregulated filament dynamics lead to pathogenesis of immune disorders.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Kinetic mechanism for viral dsRNA length discrimination by MDA5 filaments.Proc Natl Acad Sci U S A. 2012 Dec 4;109(49):E3340-9. doi: 10.1073/pnas.1208618109. Epub 2012 Nov 5. Proc Natl Acad Sci U S A. 2012. PMID: 23129641 Free PMC article.
-
Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition.Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21010-5. doi: 10.1073/pnas.1113651108. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160685 Free PMC article.
-
The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly.Mol Cell. 2014 Sep 4;55(5):771-81. doi: 10.1016/j.molcel.2014.07.003. Epub 2014 Aug 7. Mol Cell. 2014. PMID: 25127512 Free PMC article.
-
Effects of type 1 diabetes-associated IFIH1 polymorphisms on MDA5 function and expression.Curr Diab Rep. 2015 Nov;15(11):96. doi: 10.1007/s11892-015-0656-8. Curr Diab Rep. 2015. PMID: 26385483 Review.
-
Autoimmunity caused by constitutive activation of cytoplasmic viral RNA sensors.Cytokine Growth Factor Rev. 2014 Dec;25(6):739-43. doi: 10.1016/j.cytogfr.2014.08.003. Epub 2014 Aug 19. Cytokine Growth Factor Rev. 2014. PMID: 25193292 Review.
Cited by
-
The A946T variant of the RNA sensor IFIH1 mediates an interferon program that limits viral infection but increases the risk for autoimmunity.Nat Immunol. 2017 Jul;18(7):744-752. doi: 10.1038/ni.3766. Epub 2017 May 29. Nat Immunol. 2017. PMID: 28553952 Free PMC article.
-
TRIM65-catalized ubiquitination is essential for MDA5-mediated antiviral innate immunity.J Exp Med. 2017 Feb;214(2):459-473. doi: 10.1084/jem.20160592. Epub 2016 Dec 28. J Exp Med. 2017. PMID: 28031478 Free PMC article.
-
Emerging role of RNA modification N6-methyladenosine in immune evasion.Cell Death Dis. 2021 Mar 19;12(4):300. doi: 10.1038/s41419-021-03585-z. Cell Death Dis. 2021. PMID: 33741904 Free PMC article. Review.
-
Intracellular virus sensor MDA5 exacerbates vitiligo by inducing the secretion of chemokines in keratinocytes under virus invasion.Cell Death Dis. 2020 Jun 12;11(6):453. doi: 10.1038/s41419-020-2665-z. Cell Death Dis. 2020. PMID: 32532953 Free PMC article.
-
Regulation of antiviral innate immune signaling by stress-induced RNA granules.J Biochem. 2016 Mar;159(3):279-86. doi: 10.1093/jb/mvv122. Epub 2016 Jan 8. J Biochem. 2016. PMID: 26748340 Free PMC article. Review.
References
-
- Kovacsovics M, et al. Overexpression of Helicard, a CARD Containing Helicase Cleaved during Apoptosis, Accelerates DNA Degradation. Curr. Biol. 2002;12:834–843. - PubMed
-
- Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. - PubMed
-
- Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

