Aberrant lipid metabolism in anaplastic thyroid carcinoma reveals stearoyl CoA desaturase 1 as a novel therapeutic target
- PMID: 25675381
- PMCID: PMC4422887
- DOI: 10.1210/jc.2014-2764
Aberrant lipid metabolism in anaplastic thyroid carcinoma reveals stearoyl CoA desaturase 1 as a novel therapeutic target
Abstract
Context: Currently there are no efficacious therapies for patients with anaplastic thyroid carcinoma (ATC) that result in long-term disease stabilization or regression.
Objective: We sought to identify pathways critical for ATC cell progression and viability in an effort to develop new therapeutic strategies. We investigated the effects of targeted inhibition of stearoyl-CoA desaturase 1 (SCD1), a constituent of fatty acid metabolism overexpressed in ATC.
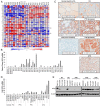
Design: A gene array of ATC and normal thyroid tissue was performed to identify gene transcripts demonstrating altered expression in tumor samples. Effects of pharmacological and the genetic inhibition of SCD1 on tumor cell viability as well as cell signaling responses to therapy were evaluated in in vitro and in vivo models of this rare, lethal malignancy.
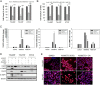
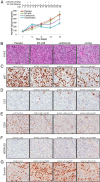
Results: The gene array analysis revealed consistent distortion of fatty acid metabolism and overexpression of SCD1 in ATC and well-differentiated thyroid carcinomas. SCD1 is critical for ATC cell survival and proliferation, the inhibition of which induced endoplasmic reticulum stress, activation of the unfolded protein response, and apoptosis. Combined suppression of endoplasmic reticulum-associated degradation, a prosurvival component of the unfolded protein response, using proteasome inhibitors resulted in a synergistic decrease in tumor cell proliferation and increased cell death.
Conclusions: SCD1 is a novel oncogenic factor specifically required for tumor cell viability in ATC. Furthermore, the expression of SCD1 appears to be correlated with thyroid tumor aggressiveness and may serve as a prognostic biomarker. These findings substantiate SCD1 as a novel tumor-specific target for therapy in patients with ATC and should be further investigated in a clinical setting.
Figures



Similar articles
-
Targeting lipid metabolism for the treatment of anaplastic thyroid carcinoma.Expert Opin Ther Targets. 2016;20(2):159-66. doi: 10.1517/14728222.2016.1086341. Epub 2015 Sep 28. Expert Opin Ther Targets. 2016. PMID: 26414044 Free PMC article. Review.
-
Exosome-mediated delivery of SCD-1 siRNA promoted the death of anaplastic thyroid carcinoma cells via regulating ROS level.Clin Transl Oncol. 2022 Feb;24(2):288-296. doi: 10.1007/s12094-021-02682-x. Epub 2021 Jul 21. Clin Transl Oncol. 2022. PMID: 34287816
-
S100A8 is a novel therapeutic target for anaplastic thyroid carcinoma.J Clin Endocrinol Metab. 2015 Feb;100(2):E232-42. doi: 10.1210/jc.2014-2988. Epub 2014 Nov 25. J Clin Endocrinol Metab. 2015. PMID: 25423568 Free PMC article.
-
Stearoyl-CoA Desaturase-1 Protects Cells against Lipotoxicity-Mediated Apoptosis in Proximal Tubular Cells.Int J Mol Sci. 2016 Nov 9;17(11):1868. doi: 10.3390/ijms17111868. Int J Mol Sci. 2016. PMID: 27834856 Free PMC article.
-
Personalized therapy in patients with anaplastic thyroid cancer: targeting genetic and epigenetic alterations.J Clin Endocrinol Metab. 2015 Jan;100(1):35-42. doi: 10.1210/jc.2014-2803. J Clin Endocrinol Metab. 2015. PMID: 25347569 Free PMC article. Review.
Cited by
-
METTL16 inhibits papillary thyroid cancer tumorigenicity through m6A/YTHDC2/SCD1-regulated lipid metabolism.Cell Mol Life Sci. 2024 Feb 9;81(1):81. doi: 10.1007/s00018-024-05146-x. Cell Mol Life Sci. 2024. PMID: 38334797 Free PMC article.
-
Fatty Acid Metabolism in Ovarian Cancer: Therapeutic Implications.Int J Mol Sci. 2022 Feb 16;23(4):2170. doi: 10.3390/ijms23042170. Int J Mol Sci. 2022. PMID: 35216285 Free PMC article. Review.
-
Down-Regulation of APTR and it's Diagnostic Value in Papillary and Anaplastic Thyroid Cancer.Pathol Oncol Res. 2020 Jan;26(1):559-565. doi: 10.1007/s12253-018-0561-y. Epub 2018 Dec 11. Pathol Oncol Res. 2020. PMID: 30539519
-
Sodium Orthovanadate Changes Fatty Acid Composition and Increased Expression of Stearoyl-Coenzyme A Desaturase in THP-1 Macrophages.Biol Trace Elem Res. 2020 Jan;193(1):152-161. doi: 10.1007/s12011-019-01699-2. Epub 2019 Mar 29. Biol Trace Elem Res. 2020. PMID: 30927246 Free PMC article.
-
Secreted Factors by Anaplastic Thyroid Cancer Cells Induce Tumor-Promoting M2-like Macrophage Polarization through a TIM3-Dependent Mechanism.Cancers (Basel). 2021 Sep 26;13(19):4821. doi: 10.3390/cancers13194821. Cancers (Basel). 2021. PMID: 34638305 Free PMC article.
References
-
- Deshpande HA, Roman S, Sosa JA. New targeted therapies and other advances in the management of anaplastic thyroid cancer. Curr Opin Oncol. 2013;25(1):44–49. - PubMed
-
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–1139. - PubMed
-
- Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103(7):1330–1335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

