The roles of Cdk5-mediated subcellular localization of FOXO1 in neuronal death
- PMID: 25673854
- PMCID: PMC4582140
- DOI: 10.1523/JNEUROSCI.3051-14.2015
The roles of Cdk5-mediated subcellular localization of FOXO1 in neuronal death
Abstract
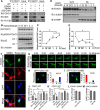
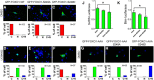
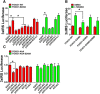
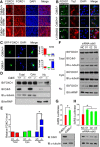
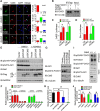
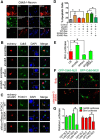
Deficiency of cyclin-dependent kinase 5 (Cdk5) has been linked to the death of postmitotic cortical neurons during brain development. We now report that, in mouse cortical neurons, Cdk5 is capable of phosphorylating the transcription factor FOXO1 at Ser249 in vitro and in vivo. Cellular stresses resulting from extracellular stimulation by H2O2 or β-amyloid promote hyperactivation of Cdk5, FOXO1 nuclear export and inhibition of its downstream transcriptional activity. In contrast, a loss of Cdk5 leads to FOXO1 translocation into the nucleus: a shift due to decreased AKT activity but independent of S249 phosphorylation. Nuclear FOXO1 upregulates transcription of the proapoptotic gene, BIM, leading to neuronal death, which can be rescued when endogenous FOXO1 was replaced by the cytoplasmically localized form of FOXO1, FOXO1-S249D. Cytoplasmic, but not nuclear, Cdk5 attenuates neuronal death by inhibiting FOXO1 transcriptional activity and BIM expression. Together, our findings suggest that Cdk5 plays a novel and unexpected role in the degeneration of postmitotic neurons through modulation of the cellular location of FOXO1, which constitutes an alternative pathway through which Cdk5 deficiency leads to neuronal death.
Keywords: Cdk5; FOXO1; neuronal death; phosphorylation.
Copyright © 2015 the authors 0270-6474/15/352624-12$15.00/0.
Figures


Similar articles
-
Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death.J Neurosci. 2005 Sep 28;25(39):8954-66. doi: 10.1523/JNEUROSCI.2899-05.2005. J Neurosci. 2005. PMID: 16192386 Free PMC article.
-
Activation of FOXO1 by Cdk1 in cycling cells and postmitotic neurons.Science. 2008 Mar 21;319(5870):1665-8. doi: 10.1126/science.1152337. Science. 2008. PMID: 18356527
-
Stabilization and activation of p53 induced by Cdk5 contributes to neuronal cell death.J Cell Sci. 2007 Jul 1;120(Pt 13):2259-71. doi: 10.1242/jcs.03468. J Cell Sci. 2007. PMID: 17591690
-
Cdk5 phosphorylation of FAK regulates centrosome-associated miocrotubules and neuronal migration.Cell Cycle. 2004 Feb;3(2):108-10. Cell Cycle. 2004. PMID: 14712065 Review.
-
Cdk5 and the non-catalytic arrest of the neuronal cell cycle.Cell Cycle. 2008 Nov 15;7(22):3487-90. doi: 10.4161/cc.7.22.7045. Epub 2008 Nov 18. Cell Cycle. 2008. PMID: 19001851 Free PMC article. Review.
Cited by
-
Epigenomic programming in early fetal brain development.Epigenomics. 2020 Jun;12(12):1053-1070. doi: 10.2217/epi-2019-0319. Epub 2020 Jul 17. Epigenomics. 2020. PMID: 32677466 Free PMC article.
-
Regulation of Bim in Health and Disease.Oncotarget. 2015 Sep 15;6(27):23058-134. doi: 10.18632/oncotarget.5492. Oncotarget. 2015. PMID: 26405162 Free PMC article. Review.
-
Intracellular Protein Shuttling: A Mechanism Relevant for Myelin Repair in Multiple Sclerosis?Int J Mol Sci. 2015 Jul 3;16(7):15057-85. doi: 10.3390/ijms160715057. Int J Mol Sci. 2015. PMID: 26151843 Free PMC article. Review.
-
Cyclin-dependent kinase 5-mediated phosphorylation of chloride intracellular channel 4 promotes oxidative stress-induced neuronal death.Cell Death Dis. 2018 Sep 20;9(10):951. doi: 10.1038/s41419-018-0983-1. Cell Death Dis. 2018. PMID: 30237421 Free PMC article.
-
Inhibition of Cdk5 induces cell death of tumor-initiating cells.Br J Cancer. 2017 Mar 28;116(7):912-922. doi: 10.1038/bjc.2017.39. Epub 2017 Feb 21. Br J Cancer. 2017. PMID: 28222068 Free PMC article.
References
-
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
