miRs-138 and -424 control palmitoylation-dependent CD95-mediated cell death by targeting acyl protein thioesterases 1 and 2 in CLL
- PMID: 25670628
- PMCID: PMC4654424
- DOI: 10.1182/blood-2014-07-586511
miRs-138 and -424 control palmitoylation-dependent CD95-mediated cell death by targeting acyl protein thioesterases 1 and 2 in CLL
Abstract
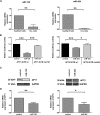
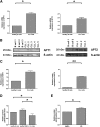
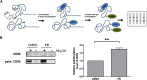
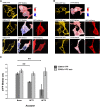
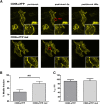
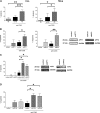
Resistance toward CD95-mediated apoptosis is a hallmark of many different malignancies, as it is known from primary chronic lymphocytic leukemia (CLL) cells. Previously, we could show that miR-138 and -424 are downregulated in CLL cells. Here, we identified 2 new target genes, namely acyl protein thioesterase (APT) 1 and 2, which are under control of both miRs and thereby significantly overexpressed in CLL cells. APTs are the only enzymes known to promote depalmitoylation. Indeed, membrane proteins are significantly less palmitoylated in CLL cells compared with normal B cells. We identified APTs to directly interact with CD95 to promote depalmitoylation, thus impairing apoptosis mediated through CD95. Specific inhibition of APTs by siRNAs, treatment with miRs-138/-424, and pharmacologic approaches restore CD95-mediated apoptosis in CLL cells and other cancer cells, pointing to an important regulatory role of APTs in CD95 apoptosis. The identification of the depalmitoylation reaction of CD95 by APTs as a microRNA (miRNA) target provides a novel molecular mechanism for how malignant cells escape from CD95-mediated apoptosis. Here, we introduce palmitoylation as a novel posttranslational modification in CLL, which might impact on localization, mobility, and function of molecules, survival signaling, and migration.
© 2015 by The American Society of Hematology.
Figures
Similar articles
-
Latent sensitivity to Fas-mediated apoptosis after CD40 ligation may explain activity of CD154 gene therapy in chronic lymphocytic leukemia.Proc Natl Acad Sci U S A. 2002 Mar 19;99(6):3854-9. doi: 10.1073/pnas.022604399. Epub 2002 Mar 12. Proc Natl Acad Sci U S A. 2002. PMID: 11891278 Free PMC article.
-
Resistance to CD95-mediated apoptosis of CD40-activated chronic lymphocytic leukemia B cells is not related to lack of DISC molecules expression.Hematol J. 2004;5(2):152-60. doi: 10.1038/sj.thj.6200362. Hematol J. 2004. PMID: 15048066
-
Role of the CD40 and CD95 (APO-1/Fas) antigens in the apoptosis of human B-cell malignancies.Br J Haematol. 1997 May;97(2):409-17. doi: 10.1046/j.1365-2141.1997.422688.x. Br J Haematol. 1997. PMID: 9163608
-
Enzymatic protein depalmitoylation by acyl protein thioesterases.Biochem Soc Trans. 2015 Apr;43(2):193-8. doi: 10.1042/BST20140235. Biochem Soc Trans. 2015. PMID: 25849916 Review.
-
Depalmitoylation and cell physiology: APT1 as a mediator of metabolic signals.Am J Physiol Cell Physiol. 2024 Apr 1;326(4):C1034-C1041. doi: 10.1152/ajpcell.00542.2023. Epub 2024 Feb 12. Am J Physiol Cell Physiol. 2024. PMID: 38344800 Review.
Cited by
-
Global miRNA profiling reveals key molecules that contribute to different chronic lymphocytic leukemia incidences in Asian and Western populations.Haematologica. 2024 Feb 1;109(2):479-492. doi: 10.3324/haematol.2023.283181. Haematologica. 2024. PMID: 37646669 Free PMC article.
-
Protein depalmitoylases.Crit Rev Biochem Mol Biol. 2018 Feb;53(1):83-98. doi: 10.1080/10409238.2017.1409191. Epub 2017 Dec 14. Crit Rev Biochem Mol Biol. 2018. PMID: 29239216 Free PMC article. Review.
-
Dysregulated MicroRNAs in Chronic Lymphocytic Leukemia.Cureus. 2024 Sep 6;16(9):e68770. doi: 10.7759/cureus.68770. eCollection 2024 Sep. Cureus. 2024. PMID: 39376808 Free PMC article. Review.
-
Elucidating the pan-oncologic landscape of S100A9: prognostic and therapeutic corollaries from an integrative bioinformatics and Mendelian randomization analysis.Sci Rep. 2024 Aug 17;14(1):19071. doi: 10.1038/s41598-024-70223-x. Sci Rep. 2024. PMID: 39154046 Free PMC article.
-
Circular RNA participates in the carcinogenesis and the malignant behavior of cancer.RNA Biol. 2017 May 4;14(5):514-521. doi: 10.1080/15476286.2015.1122162. Epub 2015 Dec 9. RNA Biol. 2017. PMID: 26649774 Free PMC article. Review.
References
-
- de Totero D, Montera M, Rosso O, et al. Resistance to CD95-mediated apoptosis of CD40-activated chronic lymphocytic leukemia B cells is not related to lack of DISC molecules expression. Hematol J. 2004;5(2):152–160. - PubMed
-
- Romano C, De Fanis U, Sellitto A, et al. Induction of CD95 upregulation does not render chronic lymphocytic leukemia B-cells susceptible to CD95-mediated apoptosis. Immunol Lett. 2005;97(1):131–139. - PubMed
-
- Dicker F, Kater AP, Fukuda T, Kipps TJ. Fas-ligand (CD178) and TRAIL synergistically induce apoptosis of CD40-activated chronic lymphocytic leukemia B cells. Blood. 2005;105(8):3193–3198. - PubMed
-
- Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407(6805):789–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

