Cytoplasmic sensing of viral nucleic acids
- PMID: 25668758
- PMCID: PMC7172233
- DOI: 10.1016/j.coviro.2015.01.012
Cytoplasmic sensing of viral nucleic acids
Abstract
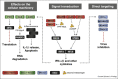
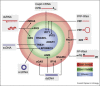
Viruses are the most abundant pathogens on earth. A fine-tuned framework of intervening pathways is in place in mammalian cells to orchestrate the cellular defence against these pathogens. Key for this system is sensor proteins that recognise specific features associated with nucleic acids of incoming viruses. Here we review the current knowledge on cytoplasmic sensors for viral nucleic acids. These sensors induce expression of cytokines, affect cellular functions required for virus replication and directly target viral nucleic acids through degradation or sequestration. Their ability to respond to a given nucleic acid is based on both the differential specificity of the individual proteins and the downstream signalling or adaptor proteins. The cooperation of these multiple proteins and pathways plays a key role in inducing successful immunity against virus infections.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Nucleic acids and endosomal pattern recognition: how to tell friend from foe?Front Cell Infect Microbiol. 2013 Jul 30;3:37. doi: 10.3389/fcimb.2013.00037. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23908972 Free PMC article. Review.
-
Recognition of viral nucleic acids in innate immunity.Rev Med Virol. 2010 Jan;20(1):4-22. doi: 10.1002/rmv.633. Rev Med Virol. 2010. PMID: 20041442 Review.
-
Camouflage and interception: how pathogens evade detection by intracellular nucleic acid sensors.Immunology. 2019 Mar;156(3):217-227. doi: 10.1111/imm.13030. Epub 2018 Dec 18. Immunology. 2019. PMID: 30499584 Free PMC article. Review.
-
Innate recognition of viruses.Immunol Lett. 2010 Jan 18;128(1):17-20. doi: 10.1016/j.imlet.2009.09.010. Epub 2009 Oct 4. Immunol Lett. 2010. PMID: 19808054 Review.
-
Nucleic Acid Sensing in Invertebrate Antiviral Immunity.Int Rev Cell Mol Biol. 2019;345:287-360. doi: 10.1016/bs.ircmb.2018.11.002. Epub 2019 Jan 3. Int Rev Cell Mol Biol. 2019. PMID: 30904195 Review.
Cited by
-
Exploiting RIG-I-like receptor pathway for cancer immunotherapy.J Hematol Oncol. 2023 Feb 8;16(1):8. doi: 10.1186/s13045-023-01405-9. J Hematol Oncol. 2023. PMID: 36755342 Free PMC article. Review.
-
Synthesis and Translation of Viral mRNA in Reovirus-Infected Cells: Progress and Remaining Questions.Viruses. 2018 Nov 27;10(12):671. doi: 10.3390/v10120671. Viruses. 2018. PMID: 30486370 Free PMC article. Review.
-
Disease Resolution in Chikungunya-What Decides the Outcome?Front Immunol. 2020 Apr 28;11:695. doi: 10.3389/fimmu.2020.00695. eCollection 2020. Front Immunol. 2020. PMID: 32411133 Free PMC article. Review.
-
Global analysis of protein-RNA interactions in SARS-CoV-2-infected cells reveals key regulators of infection.Mol Cell. 2021 Jul 1;81(13):2851-2867.e7. doi: 10.1016/j.molcel.2021.05.023. Epub 2021 May 24. Mol Cell. 2021. PMID: 34118193 Free PMC article.
-
mRNA export through an additional cap-binding complex consisting of NCBP1 and NCBP3.Nat Commun. 2015 Sep 18;6:8192. doi: 10.1038/ncomms9192. Nat Commun. 2015. PMID: 26382858 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

