A20 suppresses vascular inflammation by recruiting proinflammatory signaling molecules to intracellular aggresomes
- PMID: 25667218
- PMCID: PMC4415009
- DOI: 10.1096/fj.14-258533
A20 suppresses vascular inflammation by recruiting proinflammatory signaling molecules to intracellular aggresomes
Abstract
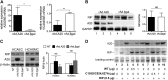
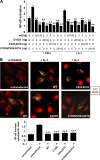
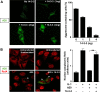
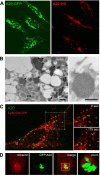
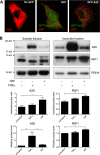
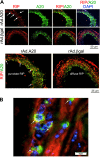
A20 protects against pathologic vascular remodeling by inhibiting the inflammatory transcription factor NF-κB. A20's function has been attributed to ubiquitin editing of receptor-interacting protein 1 (RIP1) to influence activity/stability. The validity of this mechanism was tested using a murine model of transplant vasculopathy and human cells. Mouse C57BL/6 aortae transduced with adenoviruses containing A20 (or β-galactosidase as a control) were allografted into major histocompatibility complex-mismatched BALB/c mice. Primary endothelial cells, smooth muscle cells, or transformed epithelial cells (all human) were transfected with wild-type A20 or with catalytically inactive mutants as a control. NF-κB activity and intracellular localization of RIP1 was monitored by reporter gene assay, immunofluorescent staining, and Western blotting. Native and catalytically inactive versions of A20 had similar inhibitory effects on NF-κB activity (-70% vs. -76%; P > 0.05). A20 promoted localization of RIP1 to insoluble aggresomes in murine vascular allografts and in human cells (53% vs. 0%) without altering RIP1 expression, and this process was increased by the assembly of polyubiquitin chains (87% vs. 28%; P < 0.05). A20 captures polyubiquitinated signaling intermediaries in insoluble aggresomes, thus reducing their bioavailability for downstream NF-κB signaling. This novel mechanism contributes to protection from vasculopathy in transplanted organs treated with exogenous A20.
Keywords: NF-κB; molecular mechanism; receptor interacting protein.
© FASEB.
Figures
Comment in
-
Transplant Arteriosclerosis.Transplantation. 2016 Nov;100(11):2249-2250. doi: 10.1097/TP.0000000000001440. Transplantation. 2016. PMID: 27495767 No abstract available.
Similar articles
-
A20-mediated modulation of inflammatory and immune responses in aortic allografts and development of transplant arteriosclerosis.Transplantation. 2012 Feb 27;93(4):373-82. doi: 10.1097/TP.0b013e3182419829. Transplantation. 2012. PMID: 22245872 Free PMC article.
-
Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme.Immunity. 2013 May 23;38(5):896-905. doi: 10.1016/j.immuni.2013.03.008. Epub 2013 Apr 18. Immunity. 2013. PMID: 23602765 Free PMC article.
-
Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes.Science. 2010 Feb 26;327(5969):1135-9. doi: 10.1126/science.1182364. Science. 2010. PMID: 20185725 Free PMC article.
-
A20 inhibits NF-kappaB activation by dual ubiquitin-editing functions.Trends Biochem Sci. 2005 Jan;30(1):1-4. doi: 10.1016/j.tibs.2004.11.001. Trends Biochem Sci. 2005. PMID: 15653317 Review.
-
Expression, biological activities and mechanisms of action of A20 (TNFAIP3).Biochem Pharmacol. 2010 Dec 15;80(12):2009-20. doi: 10.1016/j.bcp.2010.06.044. Epub 2010 Jul 3. Biochem Pharmacol. 2010. PMID: 20599425 Review.
Cited by
-
A20/TNFAIP3 Increases ENOS Expression in an ERK5/KLF2-Dependent Manner to Support Endothelial Cell Health in the Face of Inflammation.Front Cardiovasc Med. 2021 May 7;8:651230. doi: 10.3389/fcvm.2021.651230. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34026871 Free PMC article.
-
Plasma proteins associated with circulating carotenoids in Nepalese school-aged children.Arch Biochem Biophys. 2018 May 15;646:153-160. doi: 10.1016/j.abb.2018.03.025. Epub 2018 Mar 30. Arch Biochem Biophys. 2018. PMID: 29605494 Free PMC article. Clinical Trial.
-
Down-regulation of A20 promotes immune escape of lung adenocarcinomas.Sci Transl Med. 2021 Jul 7;13(601):eabc3911. doi: 10.1126/scitranslmed.abc3911. Sci Transl Med. 2021. PMID: 34233950 Free PMC article.
-
A prospective cohort study on serum A20 as a prognostic biomarker of aneurysmal subarachnoid hemorrhage.World J Emerg Med. 2023;14(5):360-366. doi: 10.5847/wjem.j.1920-8642.2023.079. World J Emerg Med. 2023. PMID: 37908792 Free PMC article.
-
Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop.Glia. 2018 Aug;66(8):1736-1751. doi: 10.1002/glia.23337. Epub 2018 Apr 17. Glia. 2018. PMID: 29665074 Free PMC article.
References
-
- Kunter U., Floege J., von Jürgensonn A. S., Stojanovic T., Merkel S., Gröne H. J., Ferran C. (2003) Expression of A20 in the vessel wall of rat-kidney allografts correlates with protection from transplant arteriosclerosis. Transplantation 75, 3–9 - PubMed
-
- Siracuse J. J., Fisher M. D., da Silva C. G., Peterson C. R., Csizmadia E., Moll H. P., Damrauer S. M., Studer P., Choi L. Y., Essayagh S., Kaczmarek E., Maccariello E. R., Lee A., Daniel S., Ferran C. (2012) A20-mediated modulation of inflammatory and immune responses in aortic allografts and development of transplant arteriosclerosis. Transplantation 93, 373–382 - PMC - PubMed
-
- Patel V. I., Daniel S., Longo C. R., Shrikhande G. V., Scali S. T., Czismadia E., Groft C. M., Shukri T., Motley-Dore C., Ramsey H. E., Fisher M. D., Grey S. T., Arvelo M. B., Ferran C. (2006) A20, a modulator of smooth muscle cell proliferation and apoptosis, prevents and induces regression of neointimal hyperplasia. FASEB J. 20, 1418–1430 - PubMed
-
- Xu M. Q., Yan L. N., Gou X. H., Li D. H., Huang Y. C., Hu H. Y., Wang L. Y., Han L. (2009) Zinc finger protein A20 promotes regeneration of small-for-size liver allograft and suppresses rejection and results in a longer survival in recipient rats. J. Surg. Res. 152, 35–45 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

