Synthesis of benzopentathiepin analogs and their evaluation as inhibitors of the phosphatase STEP
- PMID: 25666825
- PMCID: PMC4334692
- DOI: 10.1016/j.bmcl.2015.01.020
Synthesis of benzopentathiepin analogs and their evaluation as inhibitors of the phosphatase STEP
Abstract
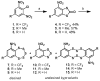
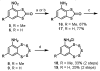
Striatal-enriched protein tyrosine phosphatase (STEP) is a brain specific protein tyrosine phosphatase that has been implicated in many neurodegenerative diseases, such as Alzheimer's disease. We recently reported the benzopentathiepin TC-2153 as a potent inhibitor of STEP in vitro, cells and animals. Herein, we report the synthesis and evaluation of TC-2153 analogs in order to define what structural features are important for inhibition and to identify positions tolerant of substitution for further study. The trifluoromethyl substitution is beneficial for inhibitor potency, and the amine is tolerant of acylation, and thus provides a convenient handle for introducing additional functionality such as reporter groups.
Keywords: Alzheimer’s disease; Inhibitor; Phosphatase; Redox; STEP.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
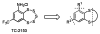
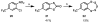
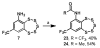
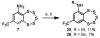
Similar articles
-
Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer's disease.PLoS Biol. 2014 Aug 5;12(8):e1001923. doi: 10.1371/journal.pbio.1001923. eCollection 2014 Aug. PLoS Biol. 2014. PMID: 25093460 Free PMC article.
-
Inhibitor of Striatal-Enriched Protein Tyrosine Phosphatase, 8-(Trifluoromethyl)-1,2,3,4,5-Benzopentathiepin-6-Amine hydrochloride (TC-2153), Produces Antidepressant-Like Effect and Decreases Functional Activity and Protein Level of 5-HT2A Receptor in the Brain.Neuroscience. 2018 Dec 1;394:220-231. doi: 10.1016/j.neuroscience.2018.10.031. Epub 2018 Oct 24. Neuroscience. 2018. PMID: 30367948
-
A novel phosphatase inhibitor may be a STEP toward ameliorating cognitive dysfunction.PLoS Biol. 2014 Aug 5;12(8):e1001924. doi: 10.1371/journal.pbio.1001924. eCollection 2014 Aug. PLoS Biol. 2014. PMID: 25093574 Free PMC article. No abstract available.
-
Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development.Future Med Chem. 2010 Oct;2(10):1563-76. doi: 10.4155/fmc.10.241. Future Med Chem. 2010. PMID: 21426149 Review.
-
PTP1B as a drug target: recent developments in PTP1B inhibitor discovery.Drug Discov Today. 2007 May;12(9-10):373-81. doi: 10.1016/j.drudis.2007.03.011. Epub 2007 Apr 6. Drug Discov Today. 2007. PMID: 17467573 Review.
Cited by
-
Synthesis and Identification of Pentathiepin-Based Inhibitors of Sporothrix brasiliensis.Antibiotics (Basel). 2019 Dec 3;8(4):249. doi: 10.3390/antibiotics8040249. Antibiotics (Basel). 2019. PMID: 31816950 Free PMC article.
-
X-ray Characterization and Structure-Based Optimization of Striatal-Enriched Protein Tyrosine Phosphatase Inhibitors.J Med Chem. 2017 Nov 22;60(22):9299-9319. doi: 10.1021/acs.jmedchem.7b01292. Epub 2017 Nov 8. J Med Chem. 2017. PMID: 29116812 Free PMC article.
-
Comprehensive Evaluation of Biological Effects of Pentathiepins on Various Human Cancer Cell Lines and Insights into Their Mode of Action.Int J Mol Sci. 2021 Jul 16;22(14):7631. doi: 10.3390/ijms22147631. Int J Mol Sci. 2021. PMID: 34299253 Free PMC article.
References
-
-
For a general overview of synaptic plasticity in neuropsychiatric disorders, see the following: Issac JT. J. Physiol. 2009;587:727. Palop JJ, Chin J, Mucke L. Nature. 2006;443:768.
-
-
-
For a prior publication on a different class of STEP inhibitors, see: Baguley TD, Xu H-C, Chatterjee M, Nairn AC, Lombroso PJ, Ellman JA. J. Med. Chem. 2013;56:7636.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

