Viroid RNA turnover: characterization of the subgenomic RNAs of potato spindle tuber viroid accumulating in infected tissues provides insights into decay pathways operating in vivo
- PMID: 25662219
- PMCID: PMC4344493
- DOI: 10.1093/nar/gkv034
Viroid RNA turnover: characterization of the subgenomic RNAs of potato spindle tuber viroid accumulating in infected tissues provides insights into decay pathways operating in vivo
Abstract
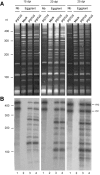
While biogenesis of viroid RNAs is well-known, how they decay is restricted to data involving host RNA silencing. Here we report an alternative degradation pathway operating on potato spindle tuber viroid (PSTVd), the type species of nuclear-replicating viroids (family Pospiviroidae). Northern-blot hybridizations with full- and partial-length probes revealed a set of PSTVd (+) subgenomic (sg)RNAs in early-infected eggplant, some partially overlapping and reaching levels comparable to those of the genomic circular and linear forms. Part of the PSTVd (+) sgRNAs were also observed in Nicotiana benthamiana (specifically in the nuclei) and tomato, wherein they have been overlooked due to their low accumulation. Primer extensions of representative (+) sgRNAs failed to detect a common 5' terminus, excluding that they could result from aborted transcription initiated at one specific site. Supporting this view, 5'- and 3'-RACE indicated that the (+) sgRNAs have 5'-OH and 3'-P termini most likely generated by RNase-mediated endonucleolytic cleavage of longer precursors. These approaches also unveiled PSTVd (-) sgRNAs with features similar to their (+) counterparts. Our results provide a mechanistic insight on how viroid decay may proceed in vivo during replication, and suggest that synthesis and decay of PSTVd strands might be coupled as in mRNA.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Replication of a pathogenic non-coding RNA increases DNA methylation in plants associated with a bromodomain-containing viroid-binding protein.Sci Rep. 2016 Oct 21;6:35751. doi: 10.1038/srep35751. Sci Rep. 2016. PMID: 27767195 Free PMC article.
-
Potato Spindle Tuber Viroid Modulates Its Replication through a Direct Interaction with a Splicing Regulator.J Virol. 2018 Sep 26;92(20):e01004-18. doi: 10.1128/JVI.01004-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30068655 Free PMC article.
-
Different rates of spontaneous mutation of chloroplastic and nuclear viroids as determined by high-fidelity ultra-deep sequencing.PLoS Pathog. 2017 Sep 14;13(9):e1006547. doi: 10.1371/journal.ppat.1006547. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28910391 Free PMC article.
-
Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation.Viruses. 2018 Sep 17;10(9):503. doi: 10.3390/v10090503. Viruses. 2018. PMID: 30227597 Free PMC article. Review.
-
Viroid pathogenesis: a critical appraisal of the role of RNA silencing in triggering the initial molecular lesion.FEMS Microbiol Rev. 2020 May 1;44(3):386-398. doi: 10.1093/femsre/fuaa011. FEMS Microbiol Rev. 2020. PMID: 32379313 Review.
Cited by
-
Highly efficient construction of infectious viroid-derived clones.Plant Methods. 2019 Aug 1;15:87. doi: 10.1186/s13007-019-0470-4. eCollection 2019. Plant Methods. 2019. PMID: 31388344 Free PMC article.
-
Elimination of Viroids from Tobacco Pollen Involves a Decrease in Propagation Rate and an Increase of the Degradation Processes.Int J Mol Sci. 2020 Apr 24;21(8):3029. doi: 10.3390/ijms21083029. Int J Mol Sci. 2020. PMID: 32344786 Free PMC article.
-
Innate Immunity Activation and RNAi Interplay in Citrus Exocortis Viroid-Tomato Pathosystem.Viruses. 2018 Oct 26;10(11):587. doi: 10.3390/v10110587. Viruses. 2018. PMID: 30373191 Free PMC article.
-
Small RNA Derived from the Virulence Modulating Region of the Potato spindle tuber viroid Silences callose synthase Genes of Tomato Plants.Plant Cell. 2015 Aug;27(8):2178-94. doi: 10.1105/tpc.15.00523. Epub 2015 Aug 19. Plant Cell. 2015. PMID: 26290537 Free PMC article.
-
Noncoding RNAs of Plant Viruses and Viroids: Sponges of Host Translation and RNA Interference Machinery.Mol Plant Microbe Interact. 2016 Mar;29(3):156-64. doi: 10.1094/MPMI-10-15-0226-FI. Epub 2016 Feb 22. Mol Plant Microbe Interact. 2016. PMID: 26900786 Free PMC article. Review.
References
-
- Diener T.O. Discovering viroids—a personal perspective. Nat. Rev. Microbiol. 2003;1:75–80. - PubMed
-
- Tabler M., Tsagris M. Viroids: petite RNA pathogens with distinguished talents. Trends Plant Sci. 2004;9:339–348. - PubMed
-
- Flores R., Hernández C., Martínez de Alba A.E., Daròs J.A., Di Serio F. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 2005;43:117–139. - PubMed
-
- Ding B. The biology of viroid-host interactions. Annu. Rev. Phytopathol. 2009;47:105–131. - PubMed
-
- Ishikawa M., Meshi T., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. A revised replication cycle for viroids: the role of longer than unit RNA in viroid replication. Mol. Gen. Gen. 1984;196:421–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

