DNA damage-induced regulatory interplay between DAXX, p53, ATM kinase and Wip1 phosphatase
- PMID: 25659035
- PMCID: PMC4353233
- DOI: 10.4161/15384101.2014.988019
DNA damage-induced regulatory interplay between DAXX, p53, ATM kinase and Wip1 phosphatase
Abstract
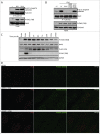
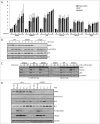
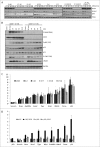
Death domain-associated protein 6 (DAXX) is a histone chaperone, putative regulator of apoptosis and transcription, and candidate modulator of p53-mediated gene expression following DNA damage. DAXX becomes phosphorylated upon DNA damage, however regulation of this modification, and its relationship to p53 remain unclear. Here we show that in human cells exposed to ionizing radiation or genotoxic drugs etoposide and neocarzinostatin, DAXX became rapidly phosphorylated in an ATM kinase-dependent manner. Our deletion and site-directed mutagenesis experiments identified Serine 564 (S564) as the dominant ATM-targeted site of DAXX, and immunofluorescence experiments revealed localization of S564-phosphorylated DAXX to PML nuclear bodies. Furthermore, using a panel of human cell types, we identified the p53-regulated Wip1 protein phosphatase as a key negative regulator of DAXX phosphorylation at S564, both in vitro and in cells. Consistent with the emerging oncogenic role of Wip1, its DAXX-dephosphorylating impact was most apparent in cancer cell lines harboring gain-of-function mutant and/or overexpressed Wip1. Unexpectedly, while Wip1 depletion increased DAXX phosphorylation both before and after DNA damage and increased p53 stability and transcriptional activity, knock-down of DAXX impacted neither p53 stabilization nor p53-mediated expression of Gadd45a, Noxa, Mdm2, p21, Puma, Sesn2, Tigar or Wip1. Consistently, analyses of cells with genetic, TALEN-mediated DAXX deletion corroborated the notion that neither phosphorylated nor non-phosphorylated DAXX is required for p53-mediated gene expression upon DNA damage. Overall, we identify ATM kinase and Wip1 phosphatase as opposing regulators of DAXX-S564 phosphorylation, and propose that the role of DAXX phosphorylation and DAXX itself are independent of p53-mediated gene expression.
Keywords: ATM; ATM, ataxia telangiectasia mutated; CHX, cycloheximide; DAXX, Death domain-associated protein 6; IR, ionizing radiation; NCS, neocarzinostatin; p21, p21WAF1/Cip1 cyclin-dependent kinase inhibitor 1; PML, promyelocytic leukemia protein; VP16, etoposide; Wi; DAXX; DNA damage; Wip1; p53.
Figures



Comment in
-
The Yin and Yang of DAXX regulation.Cell Cycle. 2015;14(3):295-6. doi: 10.1080/15384101.2015.1006552. Cell Cycle. 2015. PMID: 25651005 Free PMC article. No abstract available.
Similar articles
-
Phosphorylation of Daxx by ATM contributes to DNA damage-induced p53 activation.PLoS One. 2013;8(2):e55813. doi: 10.1371/journal.pone.0055813. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405218 Free PMC article.
-
Arsenic trioxide augments Chk2/p53-mediated apoptosis by inhibiting oncogenic Wip1 phosphatase.J Biol Chem. 2008 Jul 4;283(27):18969-79. doi: 10.1074/jbc.M800560200. Epub 2008 May 15. J Biol Chem. 2008. PMID: 18482988
-
The Wip1 Phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop.Cancer Cell. 2007 Oct;12(4):342-54. doi: 10.1016/j.ccr.2007.08.033. Cancer Cell. 2007. PMID: 17936559
-
The type 2C phosphatase Wip1: an oncogenic regulator of tumor suppressor and DNA damage response pathways.Cancer Metastasis Rev. 2008 Jun;27(2):123-35. doi: 10.1007/s10555-008-9127-x. Cancer Metastasis Rev. 2008. PMID: 18265945 Free PMC article. Review.
-
Regulation of the Wip1 phosphatase and its effects on the stress response.Front Biosci (Landmark Ed). 2012 Jan 1;17(4):1480-98. doi: 10.2741/3999. Front Biosci (Landmark Ed). 2012. PMID: 22201816 Free PMC article. Review.
Cited by
-
Enhanced UV Resistance Role of Death Domain-Associated Protein in Human MDA-MB-231 Breast Cancer Cells by Regulation of G2 DNA Damage Checkpoint.Cell Transplant. 2020 Jan-Dec;29:963689720920277. doi: 10.1177/0963689720920277. Cell Transplant. 2020. PMID: 32662684 Free PMC article.
-
DAXX-ATRX regulation of p53 chromatin binding and DNA damage response.Nat Commun. 2022 Aug 26;13(1):5033. doi: 10.1038/s41467-022-32680-8. Nat Commun. 2022. PMID: 36028493 Free PMC article.
-
An Overview of Altered Pathways Associated with Sensitivity to Platinum-Based Chemotherapy in Neuroendocrine Tumors: Strengths and Prospects.Int J Mol Sci. 2024 Aug 6;25(16):8568. doi: 10.3390/ijms25168568. Int J Mol Sci. 2024. PMID: 39201255 Free PMC article. Review.
-
MicroRNA-129-2-3p directly targets Wip1 to suppress the proliferation and invasion of intrahepatic cholangiocarcinoma.J Cancer. 2020 Mar 5;11(11):3216-3224. doi: 10.7150/jca.41492. eCollection 2020. J Cancer. 2020. PMID: 32231727 Free PMC article.
-
PPM1D in Solid and Hematologic Malignancies: Friend and Foe?Mol Cancer Res. 2022 Sep 2;20(9):1365-1378. doi: 10.1158/1541-7786.MCR-21-1018. Mol Cancer Res. 2022. PMID: 35657598 Free PMC article.
References
-
- Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 1997; 89:1067-76; PMID:9215629; http://dx.doi.org/10.1016/S0092-8674(00)80294-9 - DOI - PMC - PubMed
-
- Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1998; 281:1860-3; PMID:9743501; http://dx.doi.org/10.1126/science.281.5384.1860 - DOI - PubMed
-
- Michaelson JS, Leder P. RNAi reveals anti-apoptotic and transcriptionally repressive activities of DAXX. J Cell Sci 2003; 116:345-52; PMID:12482920; http://dx.doi.org/10.1242/jcs.00234 - DOI - PubMed
-
- Chen LY, Chen JD. Daxx silencing sensitizes cells to multiple apoptotic pathways. Mol Cell Biol 2003; 23:7108-21; PMID:14517282; http://dx.doi.org/10.1128/MCB.23.20.7108-7121.2003 - DOI - PMC - PubMed
-
- Michaelson JS, Bader D, Kuo F, Kozak C, Leder P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev 1999; 13:1918-23; PMID:10444590; http://dx.doi.org/10.1101/gad.13.15.1918 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
