MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart
- PMID: 25658461
- PMCID: PMC4452997
- DOI: 10.1097/FJC.0000000000000183
MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart
Abstract
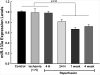
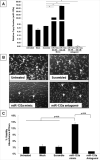
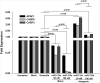
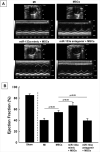
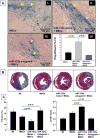
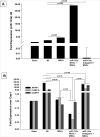
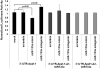
: Cardiovascular disease is the number 1 cause of morbidity and mortality in the United States. The most common manifestation of cardiovascular disease is myocardial infarction (MI), which can ultimately lead to congestive heart failure. Cell therapy (cardiomyoplasty) is a new potential therapeutic treatment alternative for the damaged heart. Recent preclinical and clinical studies have shown that mesenchymal stem cells (MSCs) are a promising cell type for cardiomyoplasty applications. However, a major limitation is the poor survival rate of transplanted stem cells in the infarcted heart. miR-133a is an abundantly expressed microRNA (miRNA) in the cardiac muscle and is downregulated in patients with MI. We hypothesized that reprogramming MSCs using miRNA mimics (double-stranded oligonucleotides) will improve survival of stem cells in the damaged heart. MSCs were transfected with miR-133a mimic and antagomirs, and the levels of miR-133a were measured by quantitative real-time polymerase chain reaction. Rat hearts were subjected to MI and MSCs transfected with miR-133a mimic or antagomir were implanted in the ischemic hearts. Four weeks after MI, cardiac function, cardiac fibrosis, miR-133a levels, and apoptosis-related genes (Apaf-1, Caspase-9, and Caspase-3) were measured in the heart. We found that transfecting MSCs with miR-133a mimic improves survival of MSCs as determined by the MTT assay. Similarly, transplantation of miR-133a mimic transfected MSCs in rat hearts subjected to MI led to a significant increase in cell engraftment, cardiac function, and decreased fibrosis when compared with MSCs only or MI groups. At the molecular level, quantitative real-time polymerase chain reaction data demonstrated a significant decrease in expression of the proapoptotic genes; Apaf-1, caspase-9, and caspase-3 in the miR-133a mimic transplanted group. Furthermore, luciferase reporter assay confirmed that miR-133a is a direct target for Apaf-1. Overall, bioengineering of stem cells through miRNAs manipulation could potentially improve the therapeutic outcome of patients undergoing stem cell transplantation for MI.
Conflict of interest statement
Figures
Similar articles
-
MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction.Stem Cell Res Ther. 2017 Nov 25;8(1):268. doi: 10.1186/s13287-017-0722-z. Stem Cell Res Ther. 2017. PMID: 29178928 Free PMC article.
-
microRNA-206 is involved in survival of hypoxia preconditioned mesenchymal stem cells through targeting Pim-1 kinase.Stem Cell Res Ther. 2016 Apr 22;7(1):61. doi: 10.1186/s13287-016-0318-z. Stem Cell Res Ther. 2016. PMID: 27103465 Free PMC article.
-
Selective inhibition of inositol hexakisphosphate kinases (IP6Ks) enhances mesenchymal stem cell engraftment and improves therapeutic efficacy for myocardial infarction.Basic Res Cardiol. 2014 Jul;109(4):417. doi: 10.1007/s00395-014-0417-x. Epub 2014 May 22. Basic Res Cardiol. 2014. PMID: 24847908
-
Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction).J Mol Cell Cardiol. 2016 May;94:107-121. doi: 10.1016/j.yjmcc.2016.03.015. Epub 2016 Apr 4. J Mol Cell Cardiol. 2016. PMID: 27056419 Review.
-
Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction.J Gene Med. 2017 Dec;19(12). doi: 10.1002/jgm.2995. Epub 2017 Dec 8. J Gene Med. 2017. PMID: 29044850 Review.
Cited by
-
Treatment of Oxidative Stress with Exosomes in Myocardial Ischemia.Int J Mol Sci. 2021 Feb 9;22(4):1729. doi: 10.3390/ijms22041729. Int J Mol Sci. 2021. PMID: 33572188 Free PMC article. Review.
-
Full-Length Dystrophin Restoration via Targeted Exon Addition in DMD-Patient Specific iPSCs and Cardiomyocytes.Int J Mol Sci. 2022 Aug 16;23(16):9176. doi: 10.3390/ijms23169176. Int J Mol Sci. 2022. PMID: 36012442 Free PMC article.
-
Using bioinformatics technology to mine the expression of serum exosomal miRNA in patients with traumatic brain injury.Front Neurosci. 2023 Apr 18;17:1145307. doi: 10.3389/fnins.2023.1145307. eCollection 2023. Front Neurosci. 2023. PMID: 37144089 Free PMC article.
-
Xenogeneic and Stem Cell-Based Therapy for Cardiovascular Diseases: Genetic Engineering of Porcine Cells and Their Applications in Heart Regeneration.Int J Mol Sci. 2020 Dec 18;21(24):9686. doi: 10.3390/ijms21249686. Int J Mol Sci. 2020. PMID: 33353186 Free PMC article. Review.
-
Circulating extracellular vesicle content reveals de novo DNA methyltransferase expression as a molecular method to predict septic shock.J Extracell Vesicles. 2019 Sep 28;8(1):1669881. doi: 10.1080/20013078.2019.1669881. eCollection 2019. J Extracell Vesicles. 2019. PMID: 31632618 Free PMC article.
References
-
- Heron M. Deaths: leading causes for 2007. Natl Vital Stat Rep. 2011 Aug 26;59(8):1–95. - PubMed
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014 Jan 21;129(3):e28–e292. - PMC - PubMed
-
- Agbulut O, Vandervelde S, Al Attar N, Larghero J, Ghostine S, Leobon B, Robidel E, Borsani P, Le Lorc'h M, Bissery A, Chomienne C, Bruneval P, Marolleau JP, Vilquin JT, Hagege A, Samuel JL, Menasche P. Comparison of human skeletal myoblasts and bone marrow-derived CD133+ progenitors for the repair of infarcted myocardium. J Am Coll Cardiol. 2004 Jul 21;44(2):458–63. - PubMed
-
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001 Apr;7(4):430–6. - PubMed
-
- Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001 Jun;938:221–9. discussion 9-30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

