Small heterodimer partner interacts with NLRP3 and negatively regulates activation of the NLRP3 inflammasome
- PMID: 25655831
- PMCID: PMC4347017
- DOI: 10.1038/ncomms7115
Small heterodimer partner interacts with NLRP3 and negatively regulates activation of the NLRP3 inflammasome
Abstract
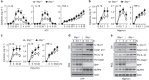
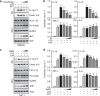
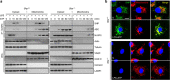
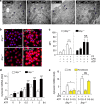
Excessive activation of the NLRP3 inflammasome results in damaging inflammation, yet the regulators of this process remain poorly defined. Herein, we show that the orphan nuclear receptor small heterodimer partner (SHP) is a negative regulator of NLRP3 inflammasome activation. NLRP3 inflammasome activation leads to an interaction between SHP and NLRP3, proteins that are both recruited to mitochondria. Overexpression of SHP competitively inhibits binding of NLRP3 to apoptosis-associated speck-like protein containing a CARD (ASC). SHP deficiency results in increased secretion of proinflammatory cytokines IL-1β and IL-18, and excessive pathologic responses typically observed in mouse models of kidney tubular necrosis and peritoneal gout. Notably, the loss of SHP results in accumulation of damaged mitochondria and a sustained interaction between NLRP3 and ASC in the endoplasmic reticulum. These data are suggestive of a role for SHP in controlling NLRP3 inflammasome activation through a mechanism involving interaction with NLRP3 and maintenance of mitochondrial homeostasis.
Figures



Similar articles
-
The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation.J Pathol. 2013 Nov;231(3):342-53. doi: 10.1002/path.4237. Epub 2013 Sep 3. J Pathol. 2013. PMID: 23843215
-
Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17254-9. doi: 10.1073/pnas.1415756111. Epub 2014 Nov 17. Proc Natl Acad Sci U S A. 2014. PMID: 25404286 Free PMC article.
-
Non-transcriptional regulation of NLRP3 inflammasome signaling by IL-4.Immunol Cell Biol. 2015 Jul;93(6):591-9. doi: 10.1038/icb.2014.125. Epub 2015 Jan 20. Immunol Cell Biol. 2015. PMID: 25601272 Free PMC article.
-
Initiation and perpetuation of NLRP3 inflammasome activation and assembly.Immunol Rev. 2015 May;265(1):35-52. doi: 10.1111/imr.12286. Immunol Rev. 2015. PMID: 25879282 Free PMC article. Review.
-
Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
Cited by
-
NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases.Front Immunol. 2021 Oct 11;12:732933. doi: 10.3389/fimmu.2021.732933. eCollection 2021. Front Immunol. 2021. PMID: 34707607 Free PMC article. Review.
-
Emerging Roles of Gut Microbial Modulation of Bile Acid Composition in the Etiology of Cardiovascular Diseases.Nutrients. 2023 Apr 12;15(8):1850. doi: 10.3390/nu15081850. Nutrients. 2023. PMID: 37111068 Free PMC article. Review.
-
The NLRP3 inflammasome in viral infection (Review).Mol Med Rep. 2023 Sep;28(3):160. doi: 10.3892/mmr.2023.13047. Epub 2023 Jul 7. Mol Med Rep. 2023. PMID: 37417336 Free PMC article. Review.
-
Progress in mass spectrometry approaches to profiling protein-protein interactions in the studies of the innate immune system.J Proteins Proteom. 2024 Sep;15(3):545-559. doi: 10.1007/s42485-024-00156-6. Epub 2024 Jun 28. J Proteins Proteom. 2024. PMID: 39380887 Free PMC article.
-
Protein kinase D at the Golgi controls NLRP3 inflammasome activation.J Exp Med. 2017 Sep 4;214(9):2671-2693. doi: 10.1084/jem.20162040. Epub 2017 Jul 17. J Exp Med. 2017. PMID: 28716882 Free PMC article.
References
-
- Yuk J. M. et al.. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat. Immunol. 12, 742–751 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

