Therapeutic genome editing: prospects and challenges
- PMID: 25654603
- PMCID: PMC4492683
- DOI: 10.1038/nm.3793
Therapeutic genome editing: prospects and challenges
Abstract
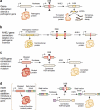
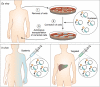
Recent advances in the development of genome editing technologies based on programmable nucleases have substantially improved our ability to make precise changes in the genomes of eukaryotic cells. Genome editing is already broadening our ability to elucidate the contribution of genetics to disease by facilitating the creation of more accurate cellular and animal models of pathological processes. A particularly tantalizing application of programmable nucleases is the potential to directly correct genetic mutations in affected tissues and cells to treat diseases that are refractory to traditional therapies. Here we discuss current progress toward developing programmable nuclease-based therapies as well as future prospects and challenges.
Figures

Similar articles
-
Recent Advances in Therapeutic Genome Editing in China.Hum Gene Ther. 2018 Feb;29(2):136-145. doi: 10.1089/hum.2017.210. Hum Gene Ther. 2018. PMID: 29446996 Review.
-
Advances in targeted genome editing.Curr Opin Chem Biol. 2012 Aug;16(3-4):268-77. doi: 10.1016/j.cbpa.2012.06.007. Epub 2012 Jul 20. Curr Opin Chem Biol. 2012. PMID: 22819644 Free PMC article. Review.
-
Therapeutic genome editing with engineered nucleases.Hamostaseologie. 2017 Jan 31;37(1):45-52. doi: 10.5482/HAMO-16-09-0035. Epub 2017 Jan 10. Hamostaseologie. 2017. PMID: 28070592 Review.
-
Targeted genome editing tools for disease modeling and gene therapy.Curr Gene Ther. 2014 Feb;14(1):2-9. doi: 10.2174/156652321402140318165450. Curr Gene Ther. 2014. PMID: 24665839 Review.
-
Genome engineering in human cells.Methods Enzymol. 2014;546:93-118. doi: 10.1016/B978-0-12-801185-0.00005-2. Methods Enzymol. 2014. PMID: 25398337 Review.
Cited by
-
Emerging Gene-editing nano-therapeutics for Cancer.Heliyon. 2024 Oct 20;10(21):e39323. doi: 10.1016/j.heliyon.2024.e39323. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39524822 Free PMC article. Review.
-
CRISPR-Cas9-mediated homology-directed repair for precise gene editing.Mol Ther Nucleic Acids. 2024 Sep 26;35(4):102344. doi: 10.1016/j.omtn.2024.102344. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39494147 Free PMC article. Review.
-
Genome editing in Sub-Saharan Africa: a game-changing strategy for climate change mitigation and sustainable agriculture.GM Crops Food. 2024 Dec 31;15(1):279-302. doi: 10.1080/21645698.2024.2411767. Epub 2024 Oct 31. GM Crops Food. 2024. PMID: 39481911 Free PMC article. Review.
-
Cardiomyopathy: pathogenesis and therapeutic interventions.MedComm (2020). 2024 Oct 25;5(11):e772. doi: 10.1002/mco2.772. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39465141 Free PMC article. Review.
-
Reporter Alleles in hiPSCs: Visual Cues on Development and Disease.Int J Mol Sci. 2024 Oct 13;25(20):11009. doi: 10.3390/ijms252011009. Int J Mol Sci. 2024. PMID: 39456792 Free PMC article. Review.
References
-
- Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187–197. - PubMed
-
- Thoene JG. Small molecule therapy for genetic disease. Cambridge University Press; Cambridge, UK ; New York: 2010.
-
- Kay MA. State-of-the-art gene-based therapies: the road ahead. Nature reviews. Genetics. 2011;12:316–328. - PubMed
-
- Gaspar HB, et al. Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Science translational medicine. 2011;3:97ra79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

