Immunologic manifestations of autophagy
- PMID: 25654553
- PMCID: PMC4350422
- DOI: 10.1172/JCI73945
Immunologic manifestations of autophagy
Abstract
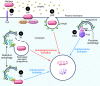
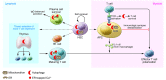
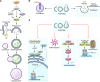
The broad immunologic roles of autophagy span innate and adaptive immunity and are often manifested in inflammatory diseases. The immune effects of autophagy partially overlap with its roles in metabolism and cytoplasmic quality control but typically expand further afield to encompass unique immunologic adaptations. One of the best-appreciated manifestations of autophagy is protection against microbial invasion, but this is by no means limited to direct elimination of intracellular pathogens and includes a stratified array of nearly all principal immunologic processes. This Review summarizes the broad immunologic roles of autophagy. Furthermore, it uses the autophagic control of Mycobacterium tuberculosis as a paradigm to illustrate the breadth and complexity of the immune effects of autophagy.
Figures
Similar articles
-
Autophagy as an immune effector against tuberculosis.Curr Opin Microbiol. 2013 Jun;16(3):355-65. doi: 10.1016/j.mib.2013.05.003. Epub 2013 Jun 18. Curr Opin Microbiol. 2013. PMID: 23790398 Free PMC article. Review.
-
Analysis of host-pathogen modulators of autophagy during Mycobacterium Tuberculosis infection and therapeutic repercussions.Int Rev Immunol. 2017 Sep 3;36(5):271-286. doi: 10.1080/08830185.2017.1356924. Epub 2017 Oct 4. Int Rev Immunol. 2017. PMID: 28976784 Review.
-
Autophagy as an innate defense against mycobacteria.Pathog Dis. 2013 Mar;67(2):108-18. doi: 10.1111/2049-632X.12023. Epub 2013 Feb 21. Pathog Dis. 2013. PMID: 23620156 Review.
-
Cell death at the cross roads of host-pathogen interaction in Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2018 Dec;113:99-121. doi: 10.1016/j.tube.2018.09.007. Epub 2018 Sep 29. Tuberculosis (Edinb). 2018. PMID: 30514519 Review.
-
Autophagy in Mycobacterium tuberculosis infection: a passepartout to flush the intruder out?Cytokine Growth Factor Rev. 2013 Aug;24(4):335-43. doi: 10.1016/j.cytogfr.2013.01.002. Epub 2013 Feb 8. Cytokine Growth Factor Rev. 2013. PMID: 23395260 Review.
Cited by
-
Photodynamic therapy: Promotion of efficacy by a sequential protocol.J Porphyr Phthalocyanines. 2016 Jan-Apr;20(1-4):302-306. doi: 10.1142/S1088424616500073. J Porphyr Phthalocyanines. 2016. PMID: 27528795 Free PMC article.
-
Genetic Variation in Autophagy-Related Genes Influences the Risk and Phenotype of Buruli Ulcer.PLoS Negl Trop Dis. 2016 Apr 29;10(4):e0004671. doi: 10.1371/journal.pntd.0004671. eCollection 2016 Apr. PLoS Negl Trop Dis. 2016. PMID: 27128681 Free PMC article.
-
Pseudomonas aeruginosa Planktonic- and Biofilm-Conditioned Media Elicit Discrete Metabolic Responses in Human Macrophages.Cells. 2020 Oct 9;9(10):2260. doi: 10.3390/cells9102260. Cells. 2020. PMID: 33050176 Free PMC article.
-
Chronic Iron Overload Results in Impaired Bacterial Killing of THP-1 Derived Macrophage through the Inhibition of Lysosomal Acidification.PLoS One. 2016 May 31;11(5):e0156713. doi: 10.1371/journal.pone.0156713. eCollection 2016. PLoS One. 2016. PMID: 27244448 Free PMC article.
-
Secretory Autophagy and Its Relevance in Metabolic and Degenerative Disease.Front Endocrinol (Lausanne). 2020 May 5;11:266. doi: 10.3389/fendo.2020.00266. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32477265 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

