The NineTeen Complex (NTC) and NTC-associated proteins as targets for spliceosomal ATPase action during pre-mRNA splicing
- PMID: 25654271
- PMCID: PMC4615276
- DOI: 10.1080/15476286.2015.1008926
The NineTeen Complex (NTC) and NTC-associated proteins as targets for spliceosomal ATPase action during pre-mRNA splicing
Abstract
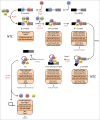
Pre-mRNA splicing is an essential step in gene expression that removes intron sequences efficiently and accurately to produce a mature mRNA for translation. It is the large and dynamic RNA-protein complex called the spliceosome that catalyzes intron removal. To carry out splicing the spliceosome not only needs to assemble correctly with the pre-mRNA but the spliceosome requires extensive remodelling of its RNA and protein components to execute the 2 steps of intron removal. Spliceosome remodelling is achieved through the action of ATPases that target both RNA and proteins to produce spliceosome conformations competent for each step of spliceosome activation, catalysis and disassembly. An increasing amount of research has pointed to the spliceosome associated NineTeen Complex (NTC) of proteins as targets for the action of a number of the spliceosomal ATPases during spliceosome remodelling. In this point-of-view article we present the latest findings on the changes in the NTC that occur following ATPase action that are required for spliceosome activation, catalysis and disassembly. We proposed that the NTC is one of the main targets of ATPase action during spliceosome remodelling required for pre-mRNA splicing.
Keywords: ATPase; Brr2; Cwc2; NineTeen Complex; PremRNA splicing; Prp16; Prp19; Prp2; Prp43; RNA helicase.
Figures
Similar articles
-
Helicases involved in splicing from malaria parasite Plasmodium falciparum.Parasitol Int. 2011 Dec;60(4):335-40. doi: 10.1016/j.parint.2011.09.007. Epub 2011 Oct 1. Parasitol Int. 2011. PMID: 21996352 Review.
-
A novel splicing factor, Yju2, is associated with NTC and acts after Prp2 in promoting the first catalytic reaction of pre-mRNA splicing.Mol Cell Biol. 2007 Aug;27(15):5403-13. doi: 10.1128/MCB.00346-07. Epub 2007 May 21. Mol Cell Biol. 2007. PMID: 17515604 Free PMC article.
-
Remodeling of U2-U6 snRNA helix I during pre-mRNA splicing by Prp16 and the NineTeen Complex protein Cwc2.Nucleic Acids Res. 2014 Jul;42(12):8008-23. doi: 10.1093/nar/gku431. Epub 2014 May 21. Nucleic Acids Res. 2014. PMID: 24848011 Free PMC article.
-
The splicing factor Prp17 interacts with the U2, U5 and U6 snRNPs and associates with the spliceosome pre- and post-catalysis.Biochem J. 2008 Dec 15;416(3):365-74. doi: 10.1042/BJ20081195. Biochem J. 2008. PMID: 18691155
-
The function of the NineTeen Complex (NTC) in regulating spliceosome conformations and fidelity during pre-mRNA splicing.Biochem Soc Trans. 2010 Aug;38(4):1110-5. doi: 10.1042/BST0381110. Biochem Soc Trans. 2010. PMID: 20659013 Free PMC article. Review.
Cited by
-
Topology of the U12-U6atac snRNA Complex of the Minor Spliceosome and Binding by NTC-Related Protein RBM22.ACS Omega. 2020 Sep 4;5(37):23549-23558. doi: 10.1021/acsomega.0c01674. eCollection 2020 Sep 22. ACS Omega. 2020. PMID: 32984674 Free PMC article.
-
Targeting the spliceosome for cutaneous squamous cell carcinoma therapy: a role for c-MYC and wild-type p53 in determining the degree of tumour selectivity.Oncotarget. 2018 May 1;9(33):23029-23046. doi: 10.18632/oncotarget.25196. eCollection 2018 May 1. Oncotarget. 2018. PMID: 29796170 Free PMC article.
-
Single molecule analysis reveals reversible and irreversible steps during spliceosome activation.Elife. 2016 May 31;5:e14166. doi: 10.7554/eLife.14166. Elife. 2016. PMID: 27244240 Free PMC article.
-
Structural and functional modularity of the U2 snRNP in pre-mRNA splicing.Crit Rev Biochem Mol Biol. 2019 Oct;54(5):443-465. doi: 10.1080/10409238.2019.1691497. Epub 2019 Nov 20. Crit Rev Biochem Mol Biol. 2019. PMID: 31744343 Free PMC article. Review.
-
An Integrated Analysis of the Identified PRPF19 as an Onco-immunological Biomarker Encompassing the Tumor Microenvironment, Disease Progression, and Prognoses in Hepatocellular Carcinoma.Front Cell Dev Biol. 2022 Feb 17;10:840010. doi: 10.3389/fcell.2022.840010. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252202 Free PMC article.
References
-
- Singh RK, Cooper TA. Pre-mRNA splicing in disease and therapeutics. Trends Mol Med 2012; 18:472-82; PMID:22819011; http://dx.doi.org/10.1016/j.molmed.2012.06.006 - DOI - PMC - PubMed
-
- Chen HC, Cheng SC. Functional roles of protein splicing factors. Biosci Rep 2012; 32:345-59; PMID:22762203; http://dx.doi.org/10.1042/BSR20120007 - DOI - PMC - PubMed
-
- Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell 2009; 136:701-18; PMID:19239890; http://dx.doi.org/10.1016/j.cell.2009.02.009 - DOI - PubMed
-
- Chan SP, Kao DI, Tsai WY, Cheng SC. The Prp19p-associated complex in spliceosome activation. Science 2003; 302:279-82; PMID:12970570; http://dx.doi.org/10.1126/science.1086602 - DOI - PubMed
-
- Hogg R, McGrail JC, O'Keefe RT. The function of the NineTeen Complex (NTC) in regulating spliceosome conformations and fidelity during pre-mRNA splicing. Biochem Soc Trans 2010; 38:1110-5; PMID:20659013; http://dx.doi.org/10.1042/BST0381110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
