Two bromodomain proteins functionally interact to recapitulate an essential BRDT-like function in Drosophila spermatocytes
- PMID: 25652540
- PMCID: PMC4345279
- DOI: 10.1098/rsob.140145
Two bromodomain proteins functionally interact to recapitulate an essential BRDT-like function in Drosophila spermatocytes
Abstract
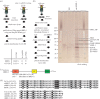
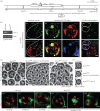
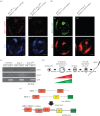
In mammals, the testis-specific bromodomain and extra terminal (BET) protein BRDT is essential for spermatogenesis. In Drosophila, it was recently reported that the tBRD-1 protein is similarly required for male fertility. Interestingly, however, tBRD-1 has two conserved bromodomains in its N-terminus but it lacks an extra terminal (ET) domain characteristic of BET proteins. Here, using proteomics approaches to search for tBRD-1 interactors, we identified tBRD-2 as a novel testis-specific bromodomain protein. In contrast to tBRD-1, tBRD-2 contains a single bromodomain, but which is associated with an ET domain in its C-terminus. Strikingly, we show that tbrd-2 knock-out males are sterile and display aberrant meiosis in a way highly similar to tbrd-1 mutants. Furthermore, these two factors co-localize and are interdependent in spermatocytes. We propose that Drosophila tBRD-1 and tBRD-2 associate into a functional BET complex in spermatocytes, which recapitulates the activity of the single mammalian BRDT-like protein.
Keywords: Drosophila; bromodomain and extra terminal family; spermatocyte; tBRD-1; tBRD-2.
Figures

Similar articles
-
tBRD-1 selectively controls gene activity in the Drosophila testis and interacts with two new members of the bromodomain and extra-terminal (BET) family.PLoS One. 2014 Sep 24;9(9):e108267. doi: 10.1371/journal.pone.0108267. eCollection 2014. PLoS One. 2014. PMID: 25251222 Free PMC article.
-
The bromodomain-containing protein tBRD-1 is specifically expressed in spermatocytes and is essential for male fertility.Biol Open. 2012 Jun 15;1(6):597-606. doi: 10.1242/bio.20121255. Epub 2012 May 9. Biol Open. 2012. PMID: 23213453 Free PMC article.
-
tBRD-1 and tBRD-2 regulate expression of genes necessary for spermatid differentiation.Biol Open. 2017 Apr 15;6(4):439-448. doi: 10.1242/bio.022467. Biol Open. 2017. PMID: 28235844 Free PMC article.
-
The role of the double bromodomain-containing BET genes during mammalian spermatogenesis.Curr Top Dev Biol. 2013;102:293-326. doi: 10.1016/B978-0-12-416024-8.00011-8. Curr Top Dev Biol. 2013. PMID: 23287038 Free PMC article. Review.
-
You bet-cha: a novel family of transcriptional regulators.Front Biosci. 2001 Aug 1;6:D1008-18. doi: 10.2741/florence. Front Biosci. 2001. PMID: 11487468 Review.
Cited by
-
Distinct spermiogenic phenotypes underlie sperm elimination in the Segregation Distorter meiotic drive system.PLoS Genet. 2021 Jul 6;17(7):e1009662. doi: 10.1371/journal.pgen.1009662. eCollection 2021 Jul. PLoS Genet. 2021. PMID: 34228705 Free PMC article.
-
The Drosophila chromosomal protein Mst77F is processed to generate an essential component of mature sperm chromatin.Open Biol. 2016 Nov;6(11):160207. doi: 10.1098/rsob.160207. Open Biol. 2016. PMID: 27810970 Free PMC article.
-
A germline PAF1 paralog complex ensures cell type-specific gene expression.Genes Dev. 2024 Oct 16;38(17-20):866-886. doi: 10.1101/gad.351930.124. Genes Dev. 2024. PMID: 39332828
-
Mitochondrial Differentiation during Spermatogenesis: Lessons from Drosophila melanogaster.Int J Mol Sci. 2024 Apr 3;25(7):3980. doi: 10.3390/ijms25073980. Int J Mol Sci. 2024. PMID: 38612789 Free PMC article. Review.
-
JQ-1 ameliorates schistosomiasis liver granuloma in mice by suppressing male and female reproductive systems and egg development of Schistosoma japonicum.PLoS Negl Trop Dis. 2022 Aug 9;16(8):e0010661. doi: 10.1371/journal.pntd.0010661. eCollection 2022 Aug. PLoS Negl Trop Dis. 2022. PMID: 35943970 Free PMC article.
References
-
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. 1999. Structure and ligand of a histone acetyltransferase bromodomain. Nature 399, 491–496 (doi:10.1038/20974) - DOI - PubMed
-
- Florence B, Faller DV. 2001. You bet-cha: a novel family of transcriptional regulators. Front. Biosci. 6, D1008–D1018 (doi:10.2741/Florence) - DOI - PubMed
-
- Lin YJ, et al. 2008. Solution structure of the extraterminal domain of the bromodomain-containing protein BRD4. Protein Sci. 17, 2174–2179 (doi:10.1110/ps.037580.108) - DOI - PMC - PubMed
-
- Belkina AC, Denis GV. 2012. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 12, 465–477 (doi:10.1038/nrc3256) - DOI - PMC - PubMed
-
- Barbieri I, Cannizzaro E, Dawson MA. 2013. Bromodomains as therapeutic targets in cancer. Brief Funct. Genomics 12, 219–230 (doi:10.1093/bfgp/elt007) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

