Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis
- PMID: 25651362
- PMCID: PMC4449790
- DOI: 10.1038/ki.2014.406
Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis
Abstract
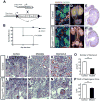
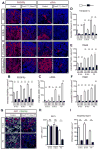
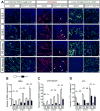
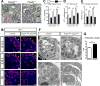
MicroRNAs, activated by the enzyme Dicer1, control post-transcriptional gene expression. Dicer1 has important roles in the epithelium during nephrogenesis, but its function in stromal cells during kidney development is unknown. To study this, we inactivated Dicer1 in renal stromal cells. This resulted in hypoplastic kidneys, abnormal differentiation of the nephron tubule and vasculature, and perinatal mortality. In mutant kidneys, genes involved in stromal cell migration and activation were suppressed as were those involved in epithelial and endothelial differentiation and maturation. Consistently, polarity of the proximal tubule was incorrect, distal tubule differentiation was diminished, and elongation of Henle's loop attenuated resulting in lack of inner medulla and papilla in stroma-specific Dicer1 mutants. Glomerular maturation and capillary loop formation were abnormal, whereas peritubular capillaries, with enhanced branching and increased diameter, formed later. In Dicer1-null renal stromal cells, expression of factors associated with migration, proliferation, and morphogenic functions including α-smooth muscle actin, integrin-α8, -β1, and the WNT pathway transcriptional regulator LEF1 were reduced. Dicer1 mutation in stroma led to loss of expression of distinct microRNAs. Of these, miR-214, -199a-5p, and -199a-3p regulate stromal cell functions ex vivo, including WNT pathway activation, migration, and proliferation. Thus, Dicer1 activity in the renal stromal compartment regulates critical stromal cell functions that, in turn, regulate differentiation of the nephron and vasculature during nephrogenesis.
Figures

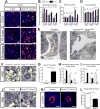
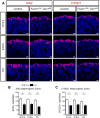
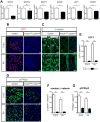
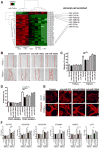
Comment in
-
Size doesn't matter; in the stroma, little things make all the difference.Kidney Int. 2015 Jun;87(6):1087-9. doi: 10.1038/ki.2015.77. Kidney Int. 2015. PMID: 26024024
Similar articles
-
Size doesn't matter; in the stroma, little things make all the difference.Kidney Int. 2015 Jun;87(6):1087-9. doi: 10.1038/ki.2015.77. Kidney Int. 2015. PMID: 26024024
-
MOV10 binding circ-DICER1 regulates the angiogenesis of glioma via miR-103a-3p/miR-382-5p mediated ZIC4 expression change.J Exp Clin Cancer Res. 2019 Jan 8;38(1):9. doi: 10.1186/s13046-018-0990-1. J Exp Clin Cancer Res. 2019. PMID: 30621721 Free PMC article.
-
A Sall1-NuRD interaction regulates multipotent nephron progenitors and is required for loop of Henle formation.Development. 2017 Sep 1;144(17):3080-3094. doi: 10.1242/dev.148692. Epub 2017 Jul 31. Development. 2017. PMID: 28760814 Free PMC article.
-
Induction and patterning of the metanephric nephron.Semin Cell Dev Biol. 2014 Dec;36:31-8. doi: 10.1016/j.semcdb.2014.08.014. Epub 2014 Sep 3. Semin Cell Dev Biol. 2014. PMID: 25194660 Free PMC article. Review.
-
Patterning the renal vascular bed.Semin Cell Dev Biol. 2014 Dec;36:50-6. doi: 10.1016/j.semcdb.2014.08.002. Epub 2014 Aug 13. Semin Cell Dev Biol. 2014. PMID: 25128732 Free PMC article. Review.
Cited by
-
Netrin 1 directs vascular patterning and maturity in the developing kidney.Development. 2023 Nov 15;150(22):dev201886. doi: 10.1242/dev.201886. Epub 2023 Nov 22. Development. 2023. PMID: 37818607 Free PMC article.
-
Epigenetic regulation of kidney progenitor cells.Stem Cells Transl Med. 2020 Jun;9(6):655-660. doi: 10.1002/sctm.19-0289. Epub 2020 Mar 12. Stem Cells Transl Med. 2020. PMID: 32163228 Free PMC article. Review.
-
The Lhx1-Ldb1 complex interacts with Furry to regulate microRNA expression during pronephric kidney development.Sci Rep. 2018 Oct 30;8(1):16029. doi: 10.1038/s41598-018-34038-x. Sci Rep. 2018. PMID: 30375416 Free PMC article.
-
Gestational Low Protein Diet Modulation on miRNA Transcriptome and Its Target During Fetal and Breastfeeding Nephrogenesis.Front Physiol. 2021 Jun 22;12:648056. doi: 10.3389/fphys.2021.648056. eCollection 2021. Front Physiol. 2021. PMID: 34239447 Free PMC article.
-
MicroRNAs as Master Regulators of Glomerular Function in Health and Disease.J Am Soc Nephrol. 2017 Jun;28(6):1686-1696. doi: 10.1681/ASN.2016101117. Epub 2017 Feb 23. J Am Soc Nephrol. 2017. PMID: 28232619 Free PMC article. Review.
References
-
- Levinson RS, Levinson RS, Batourina E, et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. - PubMed
-
- Hatini V, Hatini V, Huh SO, et al. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

