Synthesis of novel triazoles, tetrazine, thiadiazoles and their biological activities
- PMID: 25648599
- PMCID: PMC6272378
- DOI: 10.3390/molecules20022591
Synthesis of novel triazoles, tetrazine, thiadiazoles and their biological activities
Abstract
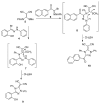
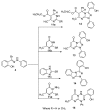
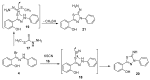
An expedient synthesis of novel triazoles, tetrazine and thiadiazoles, using conveniently accessible and commercially available starting materials has been achieved. The synthesized compounds were characterized by spectroscopic and elemental analyses, and screened for their antibacterial activities against four different strains, namely E. coli, P. aeruginosa, S. aureus and B. megaterium. In particular, the compounds 5, 24 and 26h exhibited excellent antibacterial activities compared to the reference antibiotic. To get further insight about their behavior, these compounds were tested for their antioxidant activities via SOD-like activity, DPPH free radical scavenging activity, ABST and NO, which showed promising results. Furthermore, these compounds effectively promoted the cleavage of genomic DNA as well, in the absence of any external additives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
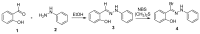
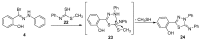
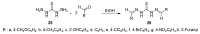

Similar articles
-
[Synthesis and antibacterial activities of 3,6-disubstituted-5,6-dihydrogen-1,2,4-s-triazolo-[3,4-b]-1,3,4-thiadiazoles].Yao Xue Xue Bao. 2001 Apr;36(4):310-2. Yao Xue Xue Bao. 2001. PMID: 12580064 Chinese.
-
[Synthesis and antibacterial activity of 3-(4-piperazin-1-yl-phenyl)-s-triazolo [3,4-b] [1,3,4] thiadiazole hydrochlorides].Yao Xue Xue Bao. 2007 Jan;42(1):54-7. Yao Xue Xue Bao. 2007. PMID: 17520807 Chinese.
-
Synthesis of some new [1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and [1,2,4]triazolo[3,4-b][1,3,4] thiadiazoles starting from 5-nitro-2-furoic acid and evaluation of their antimicrobial activity.Bioorg Med Chem. 2011 Aug 1;19(15):4506-12. doi: 10.1016/j.bmc.2011.06.024. Epub 2011 Jun 16. Bioorg Med Chem. 2011. PMID: 21723735
-
Synthesis and antibacterial activities of novel imidazo[2,1-b]-1,3,4-thiadiazoles.Molecules. 2011 Jun 28;16(7):5496-506. doi: 10.3390/molecules16075496. Molecules. 2011. PMID: 21712761 Free PMC article.
-
Synthesis and antimicrobial activity of pyrimidinyl 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles.Eur J Med Chem. 2018 Feb 10;145:1-10. doi: 10.1016/j.ejmech.2017.12.067. Epub 2017 Dec 21. Eur J Med Chem. 2018. PMID: 29310025
Cited by
-
Utilization of Cyanoacetohydrazide and Oxadiazolyl Acetonitrile in the Synthesis of Some New Cytotoxic Heterocyclic Compounds.Molecules. 2016 Jan 29;21(2):155. doi: 10.3390/molecules21020155. Molecules. 2016. PMID: 26840279 Free PMC article.
-
New Polymer Inclusion Membranes in the Separation of Palladium, Zinc and Nickel Ions from Aqueous Solutions.Polymers (Basel). 2021 Apr 28;13(9):1424. doi: 10.3390/polym13091424. Polymers (Basel). 2021. PMID: 33925085 Free PMC article.
-
Synthesis, antimicrobial, and antiproliferative activities of substituted phenylfuranylnicotinamidines.Drug Des Devel Ther. 2016 Mar 11;10:1133-46. doi: 10.2147/DDDT.S102128. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27042005 Free PMC article.
References
-
- Liu X.H., Tan C.X., Weng J.Q. Phase transfer-catalyzed, one-pot synthesis of some novel N-pyrimidinyl-N'-nicotinylthiourea derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2011;186:552–557. doi: 10.1080/10426507.2010.508059. - DOI
-
- Su N.N., Li Y., Yu S.J., Zhang X., Liu X.H., Zhao W.G. Microwave-assisted synthesis of some novel 1,2,3-triazoles by click chemistry, and their biological activity. Res. Chem. Intermed. 2013;39:759–766. doi: 10.1007/s11164-012-0595-9. - DOI
-
- Liu X.H., Pan L., Tan C.X., Weng J.Q., Wang B.L., Li Z.M. Synthesis, crystal structure, bioactivity and DFT calculation of new oxime ester derivatives containing cyclopropane moiety. Pestic. Biochem. Physiol. 2011;101:143–147. doi: 10.1016/j.pestbp.2011.08.006. - DOI
-
- Liu X.H., Pan L., Ma Y., Weng J.Q., Tan C.X., Li Y.H., Shi Y.X., Li B.J., Li Z.M., Zhang Y.G. Design, synthesis, biological activities, and 3D-QSAR of new N,N'-diacylhydrazines containing 2-(2,4-dichlorophenoxy)propane moiety. Chem. Biol. Drug Des. 2011;78:689–694. doi: 10.1111/j.1747-0285.2011.01205.x. - DOI - PubMed
-
- Chen P.Q., Tan C.X., Weng J.Q., Liu X.H. Synthesis, structure and DFT calculation of chlorimuron-ethyl. Asian J. Chem. 2012;24:2808–2810.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

